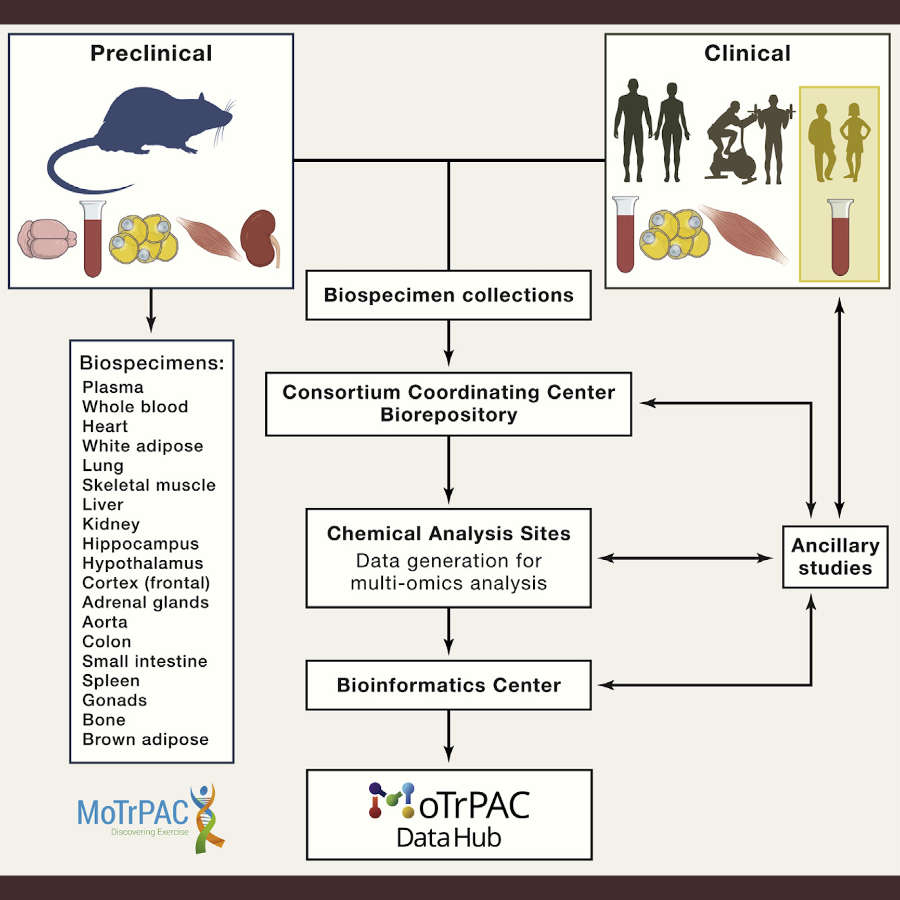
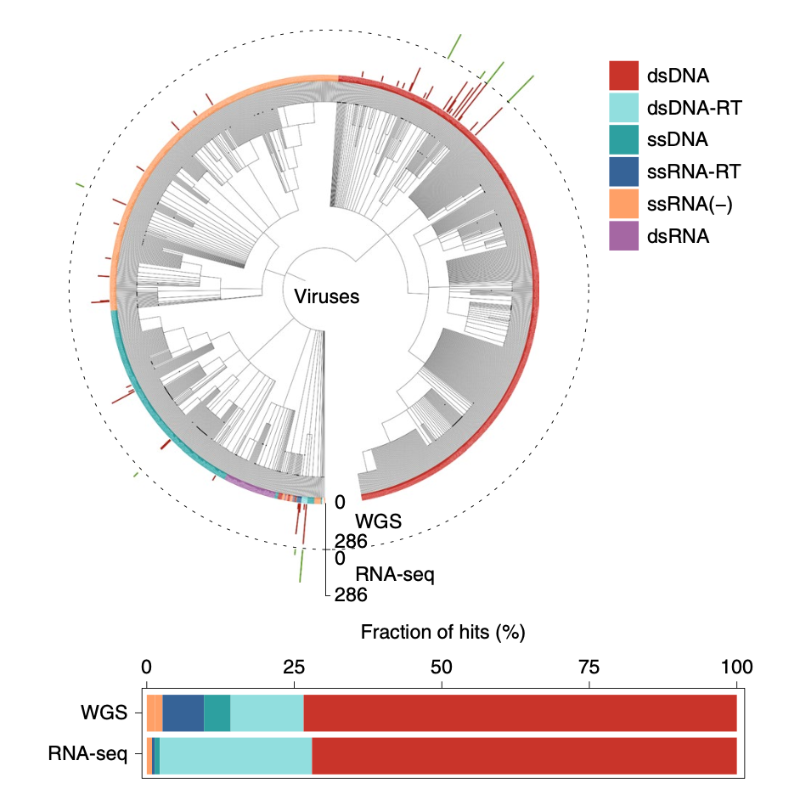
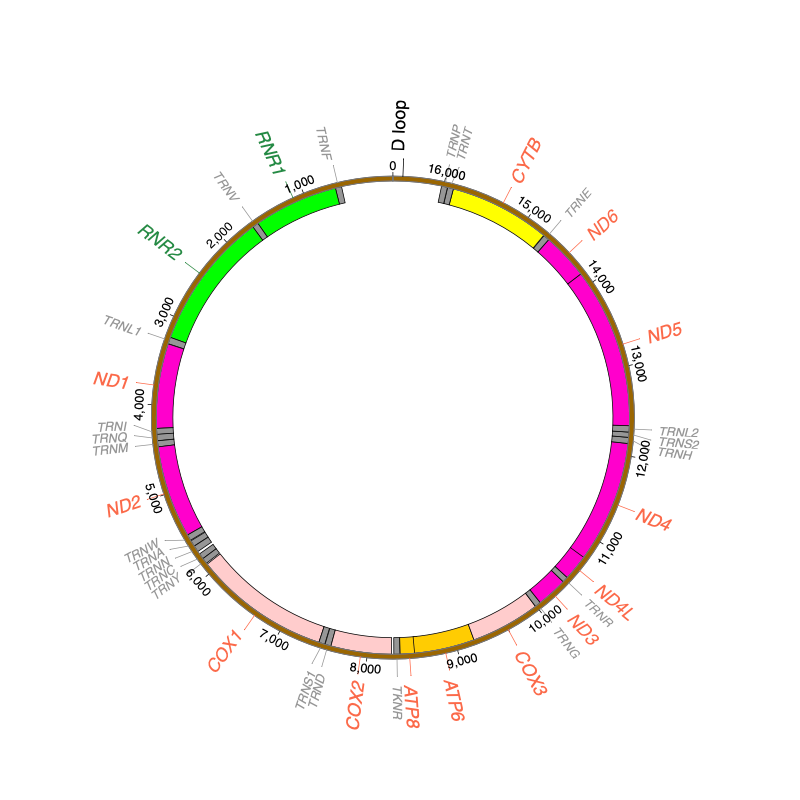
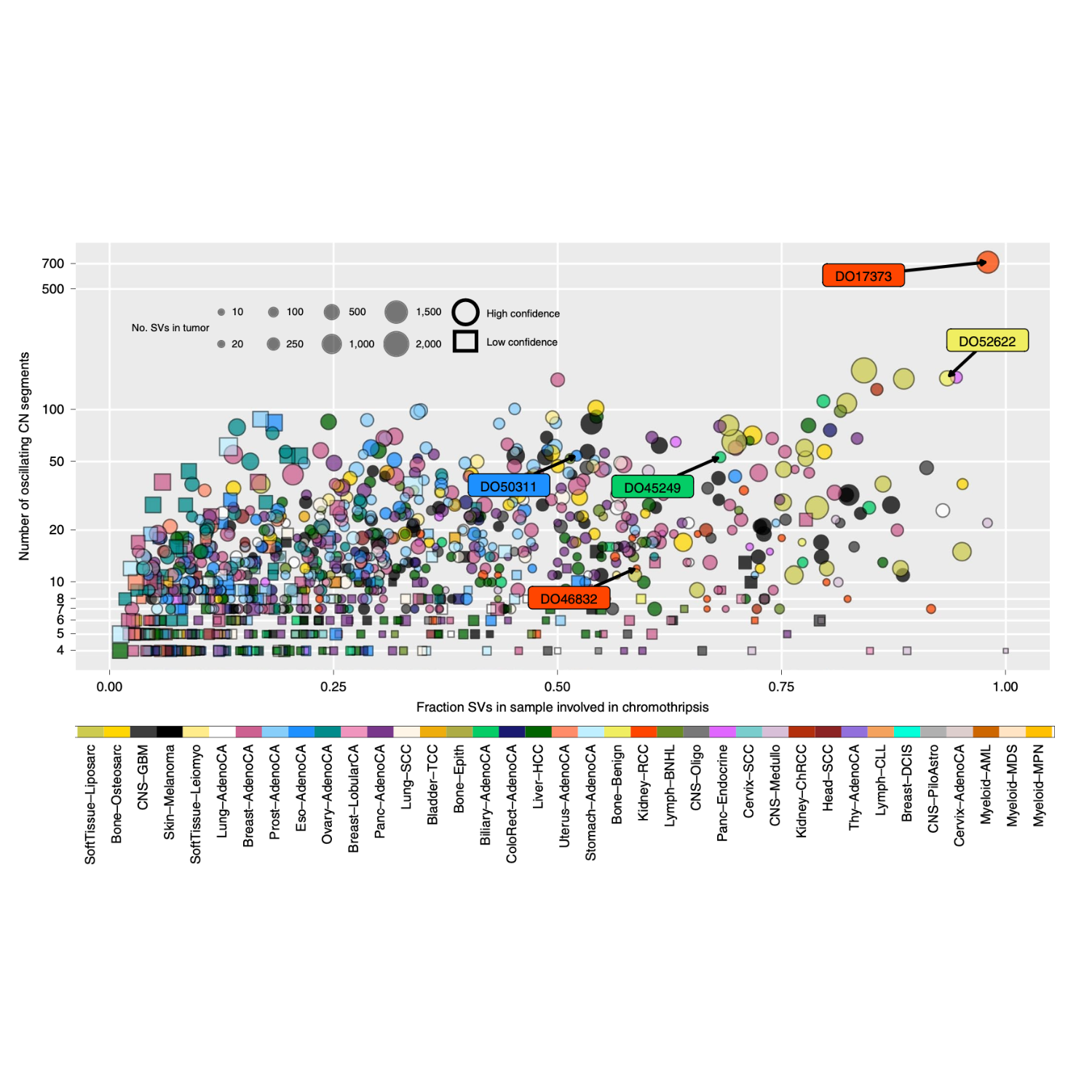
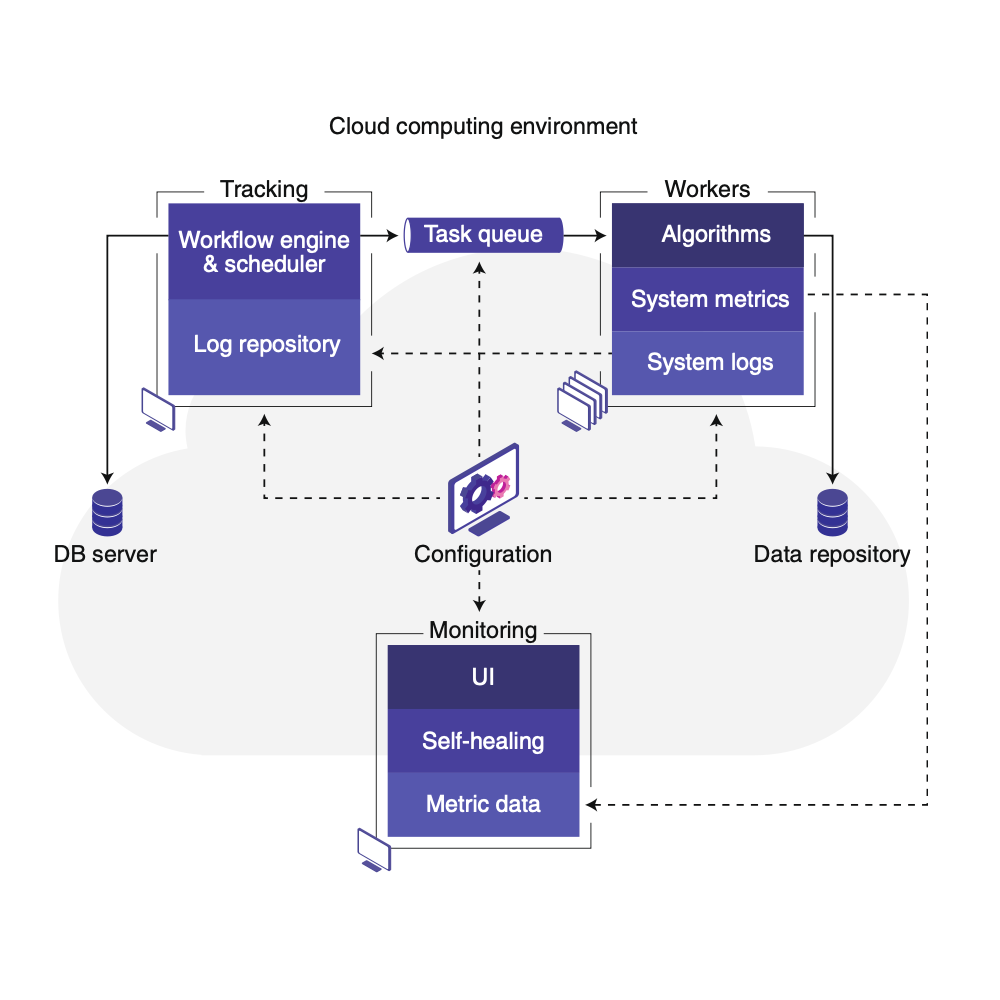
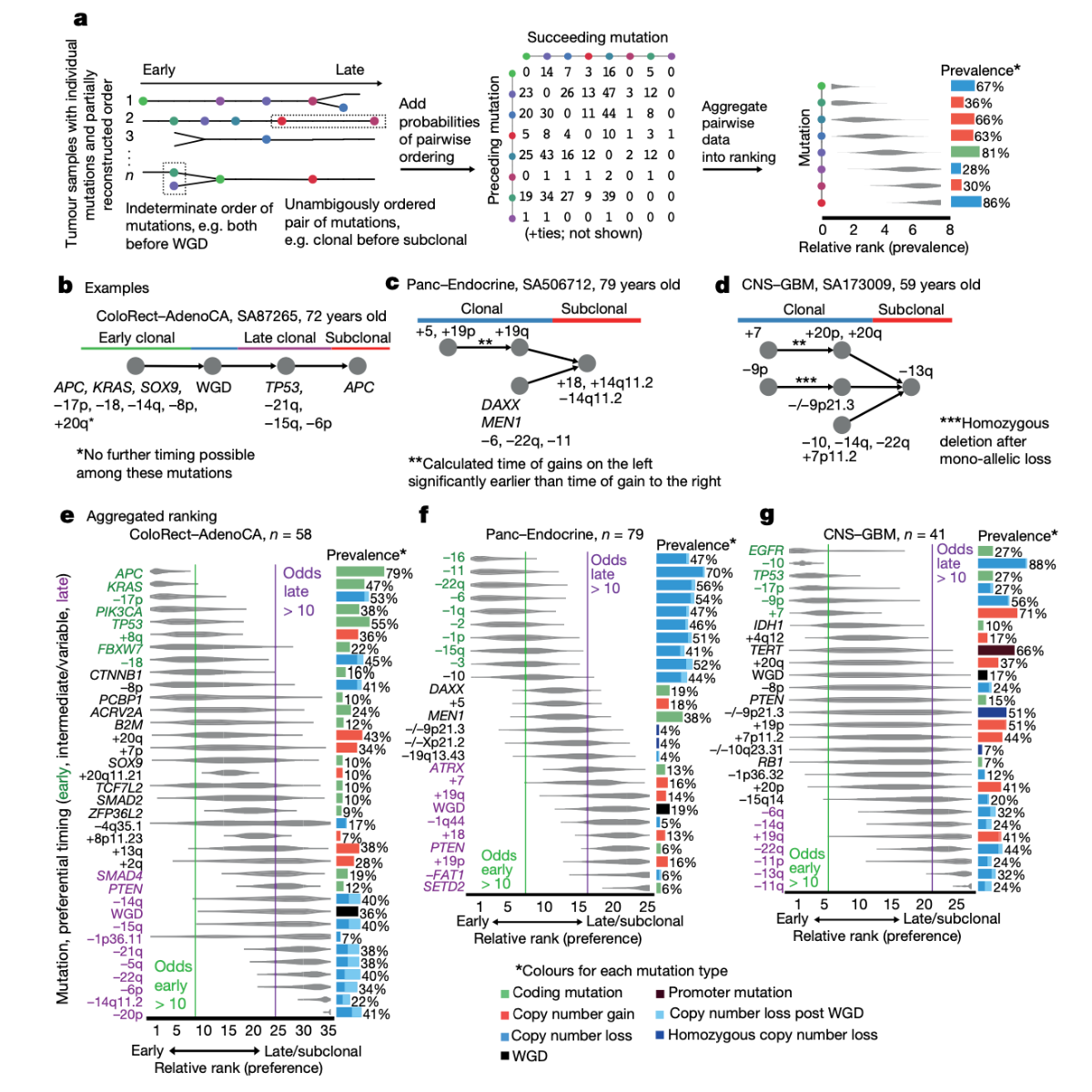
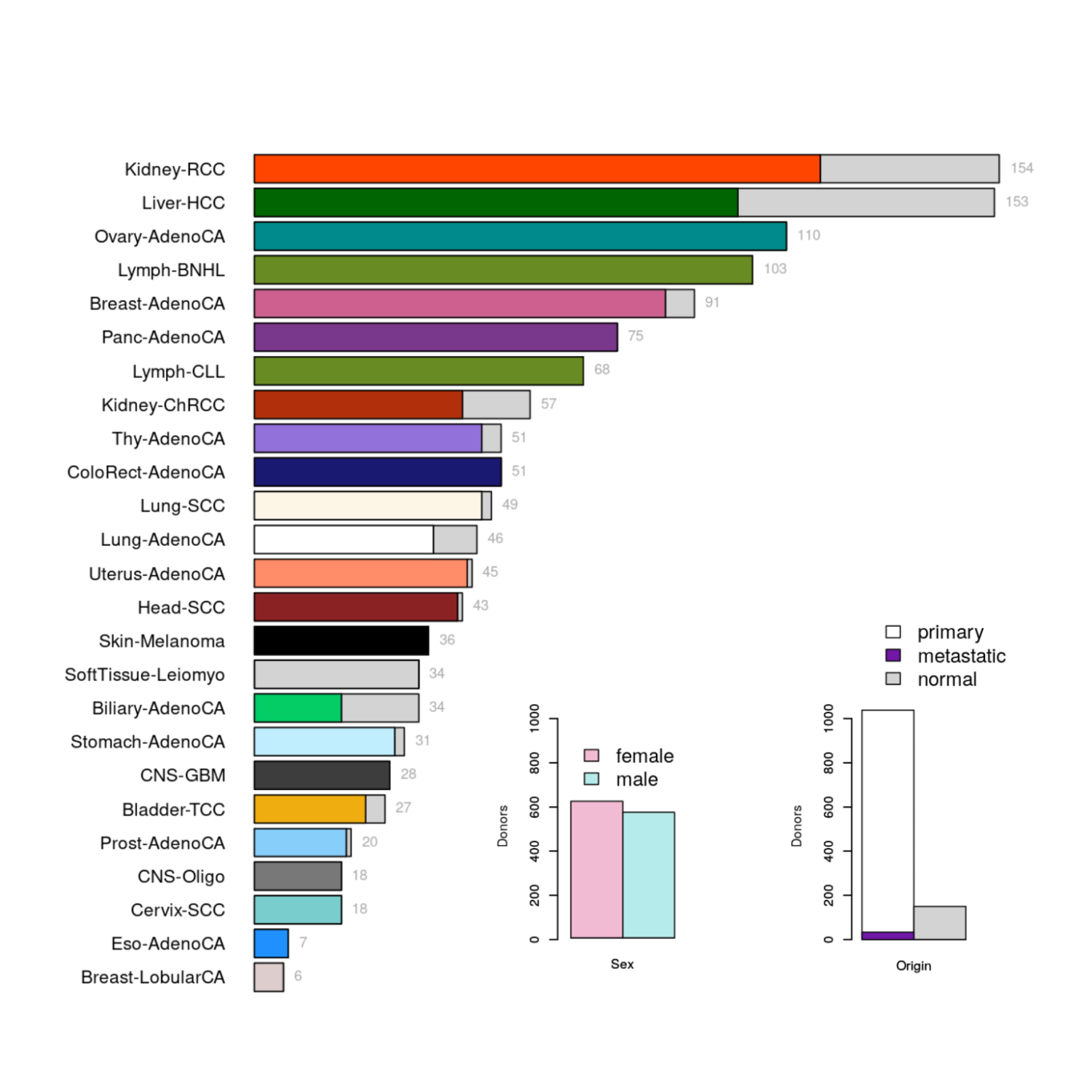
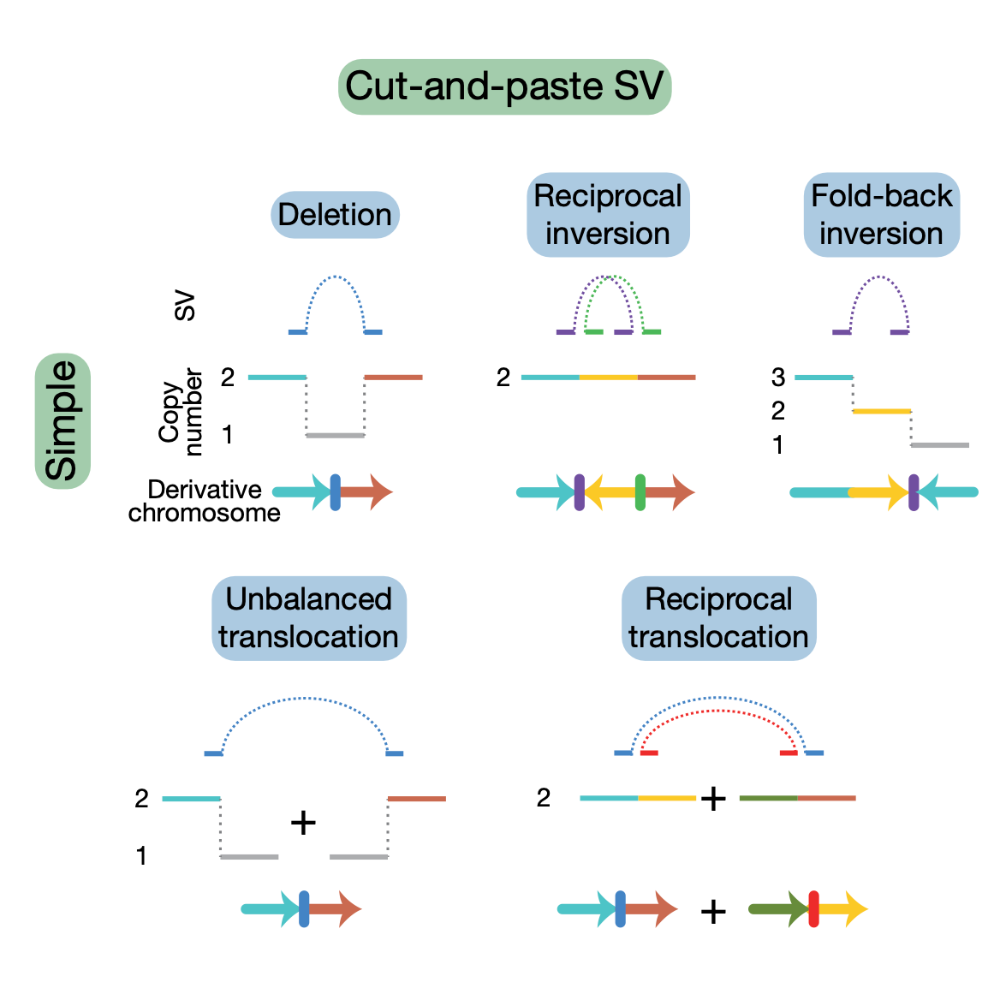
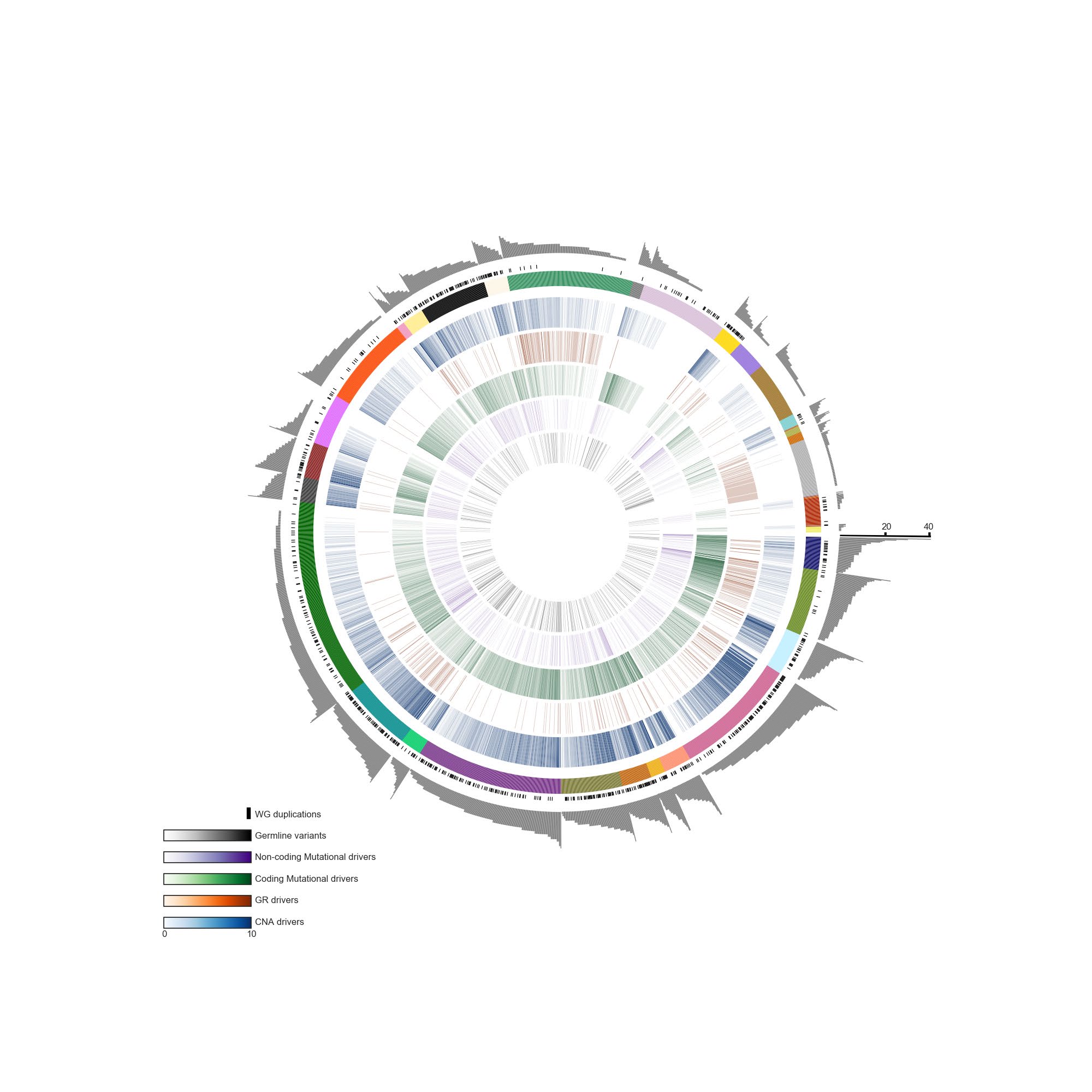
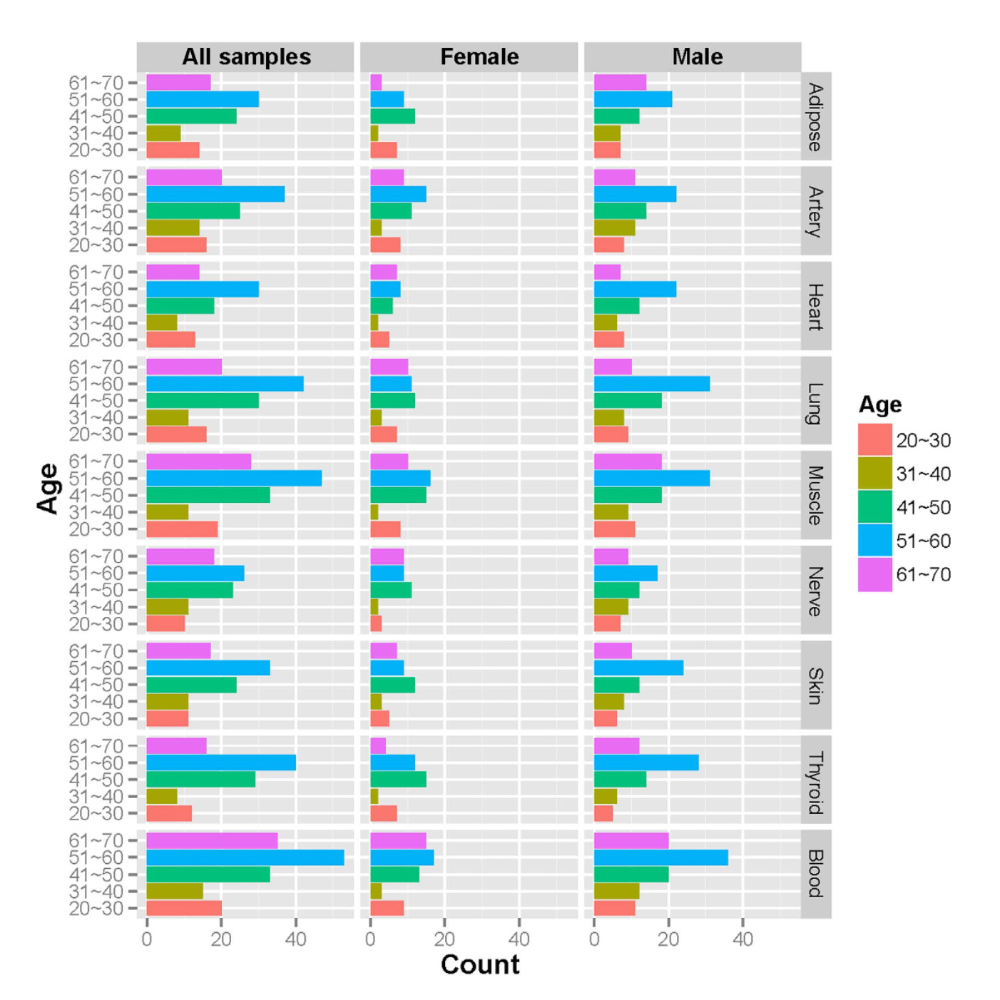
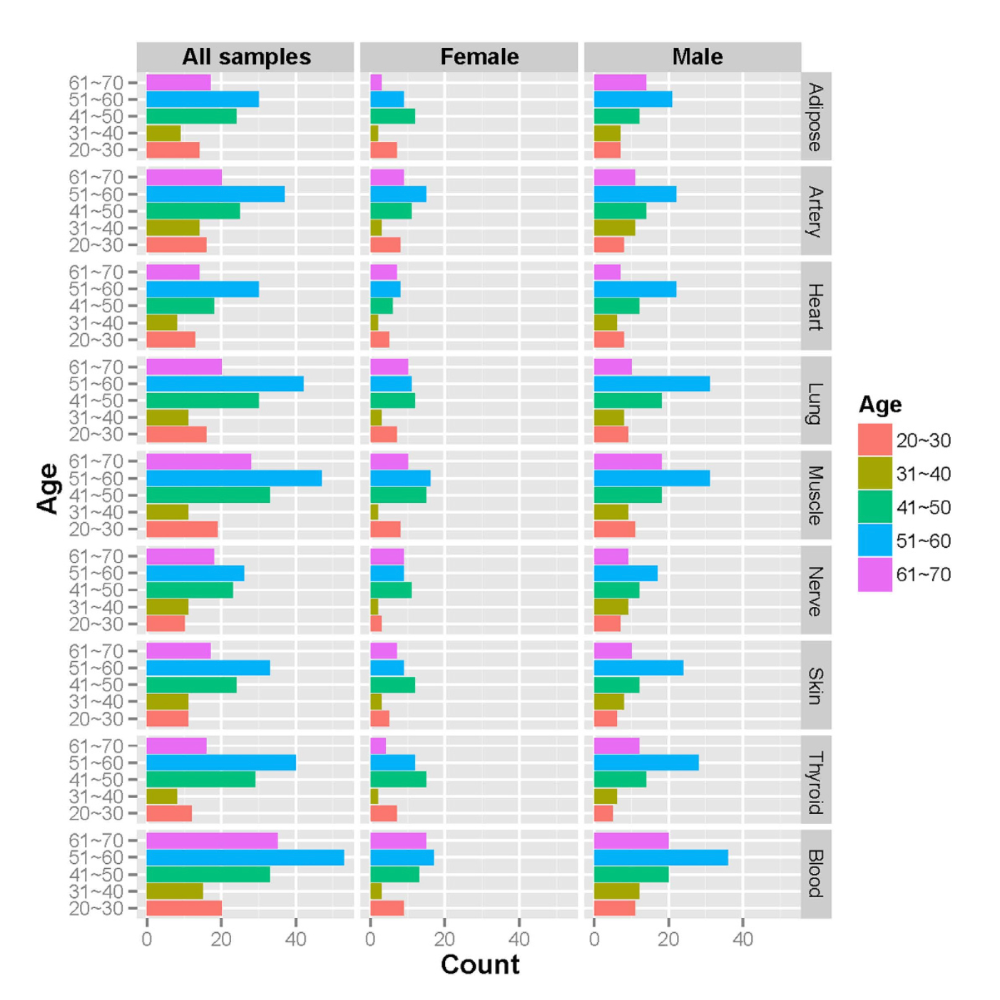
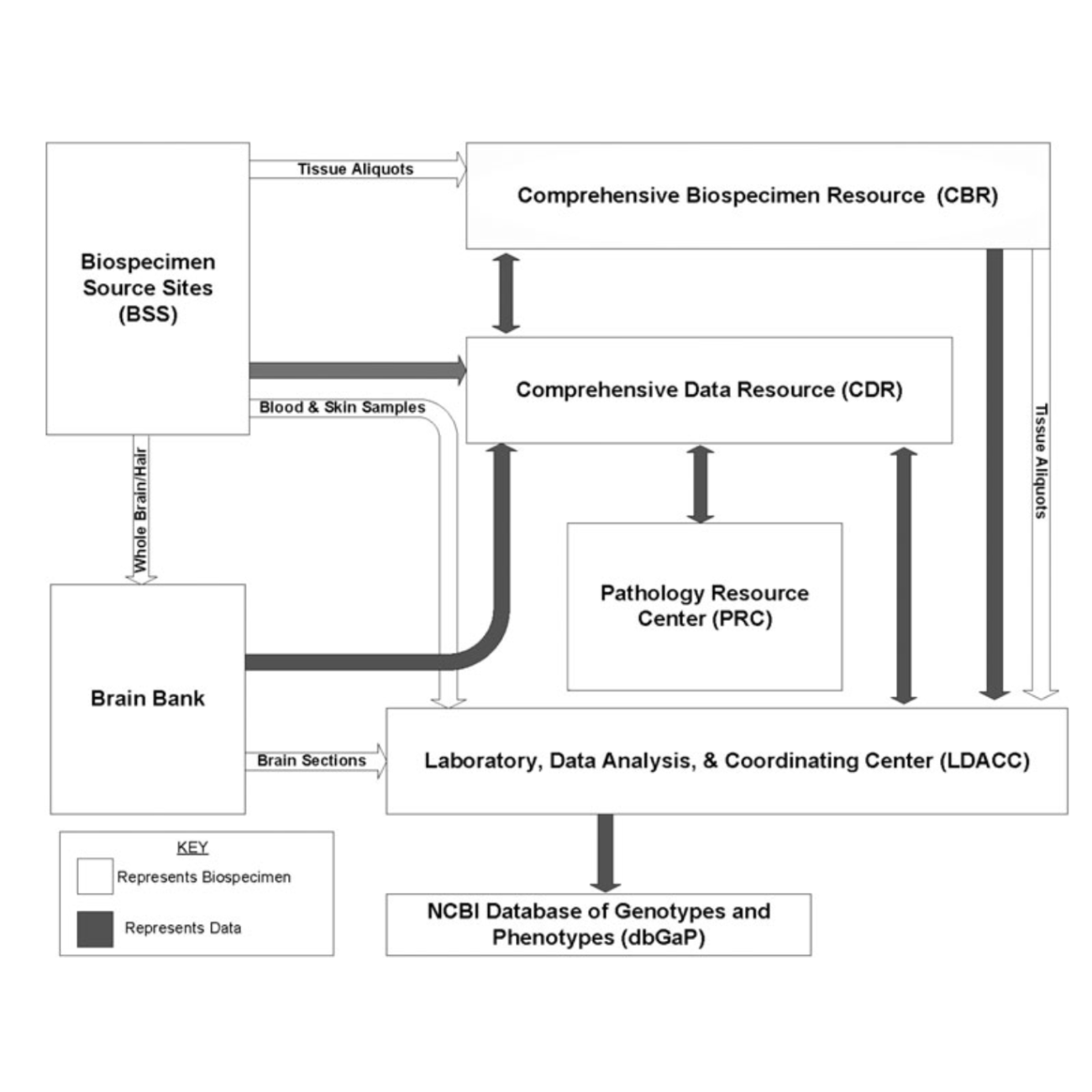
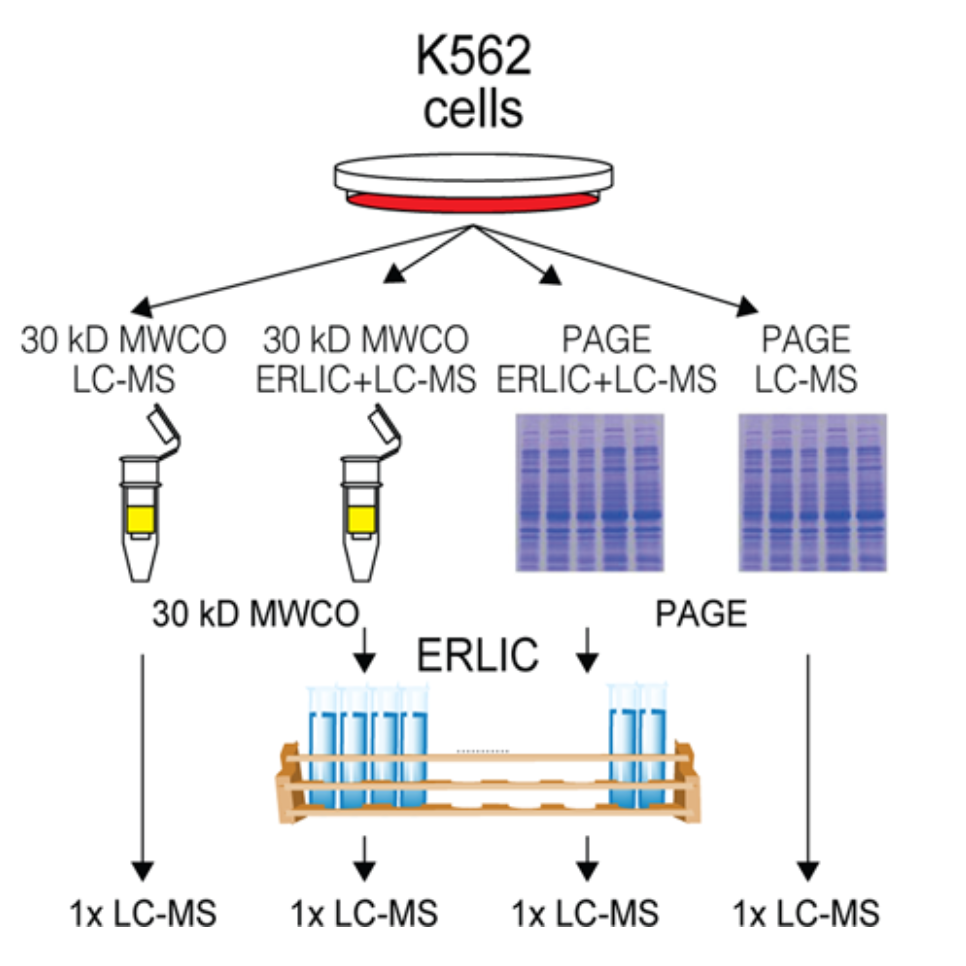
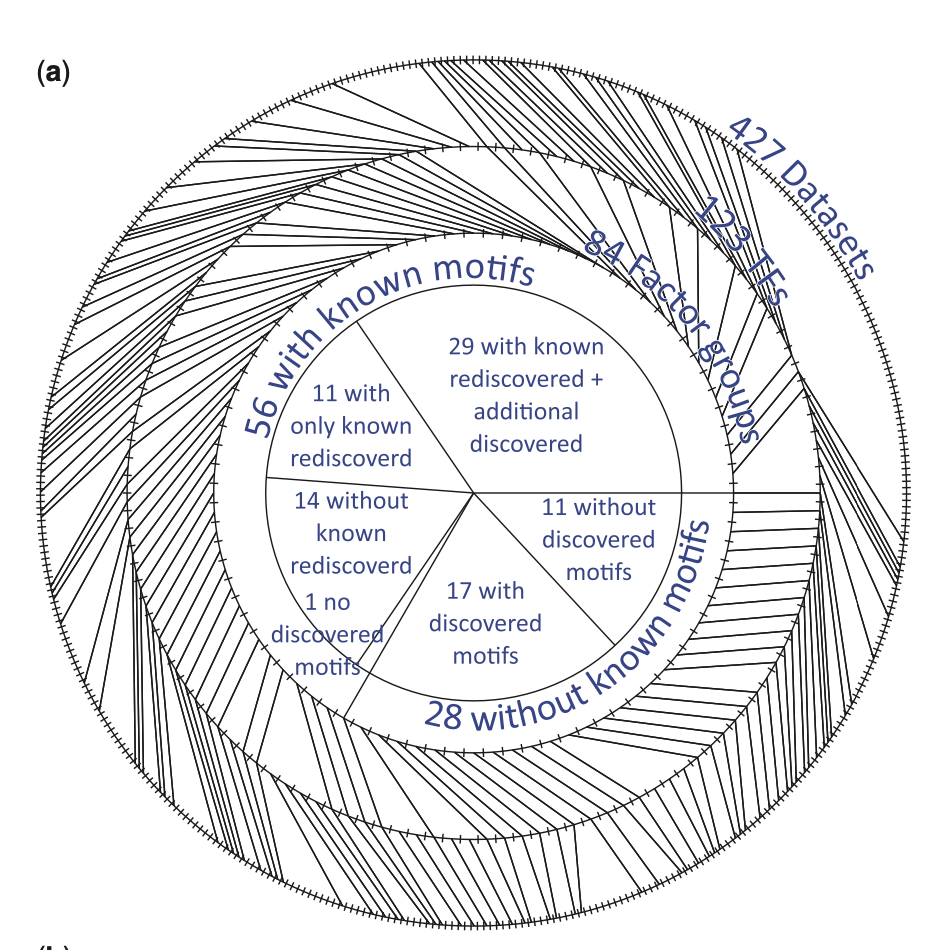
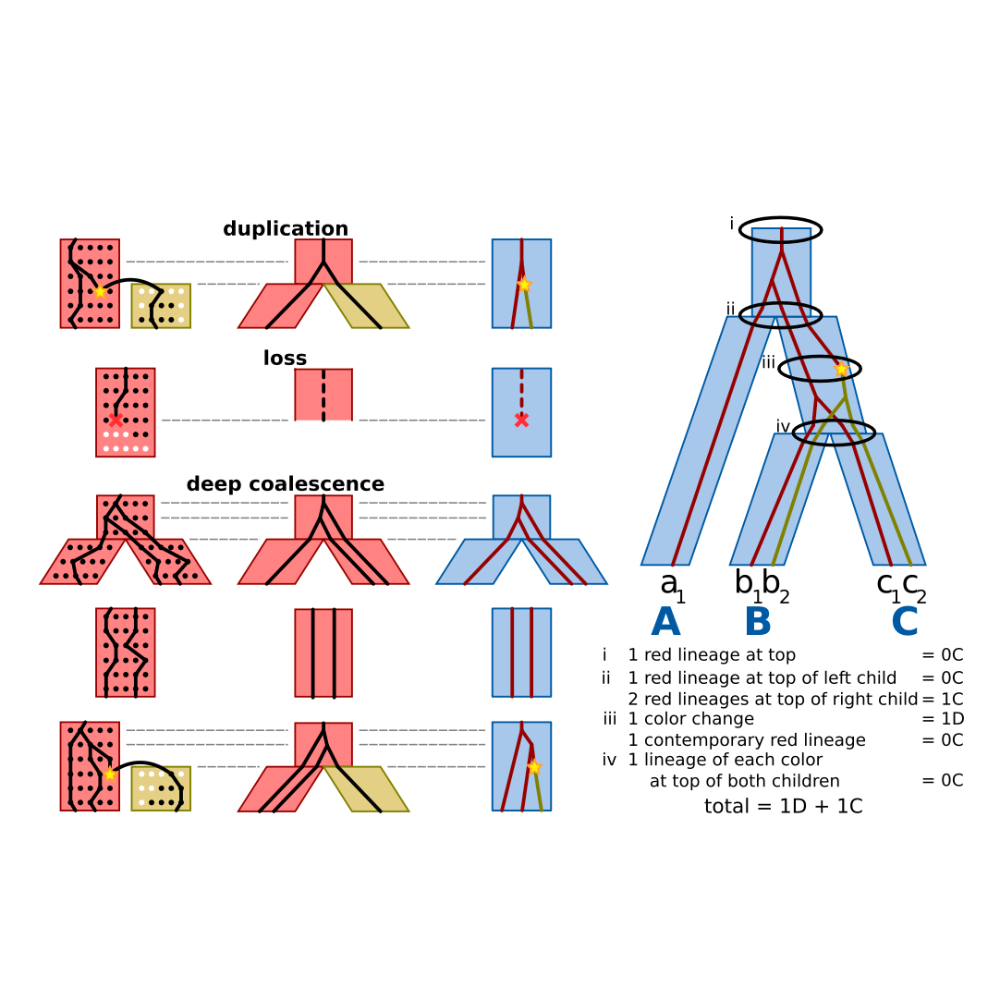
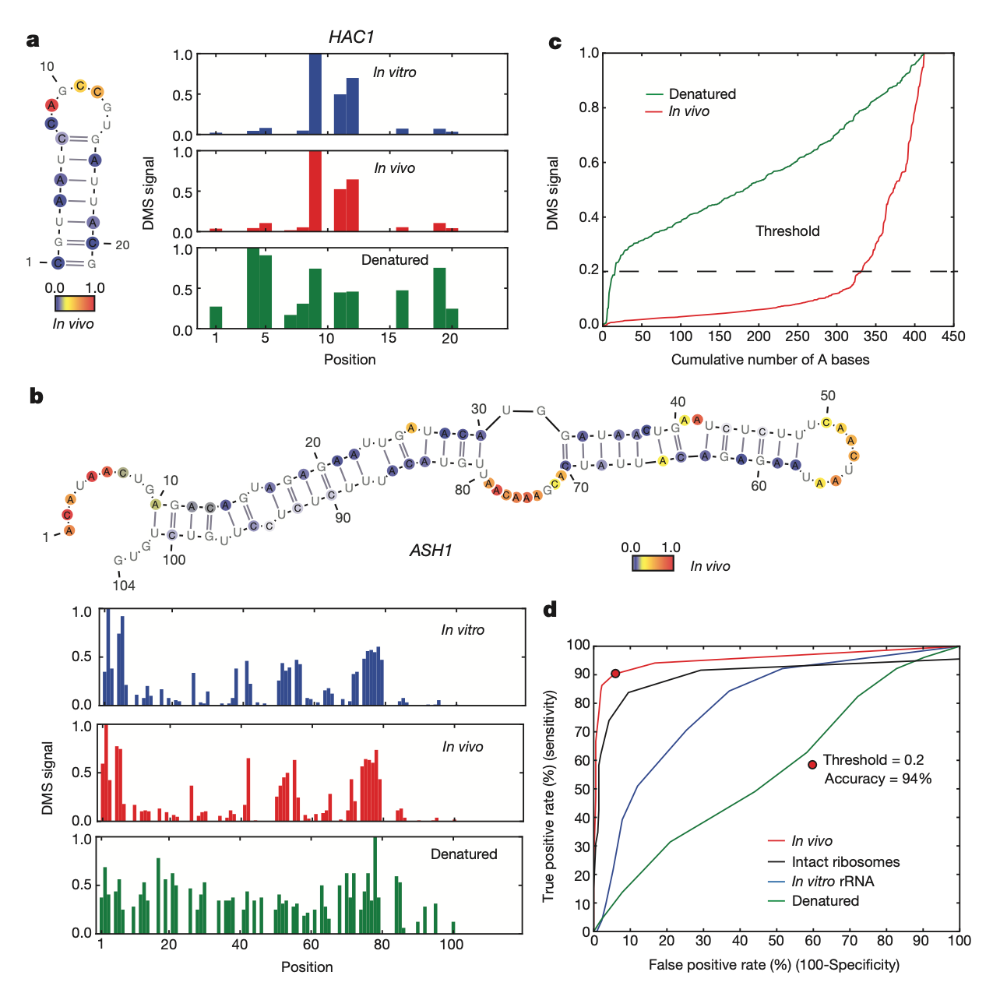
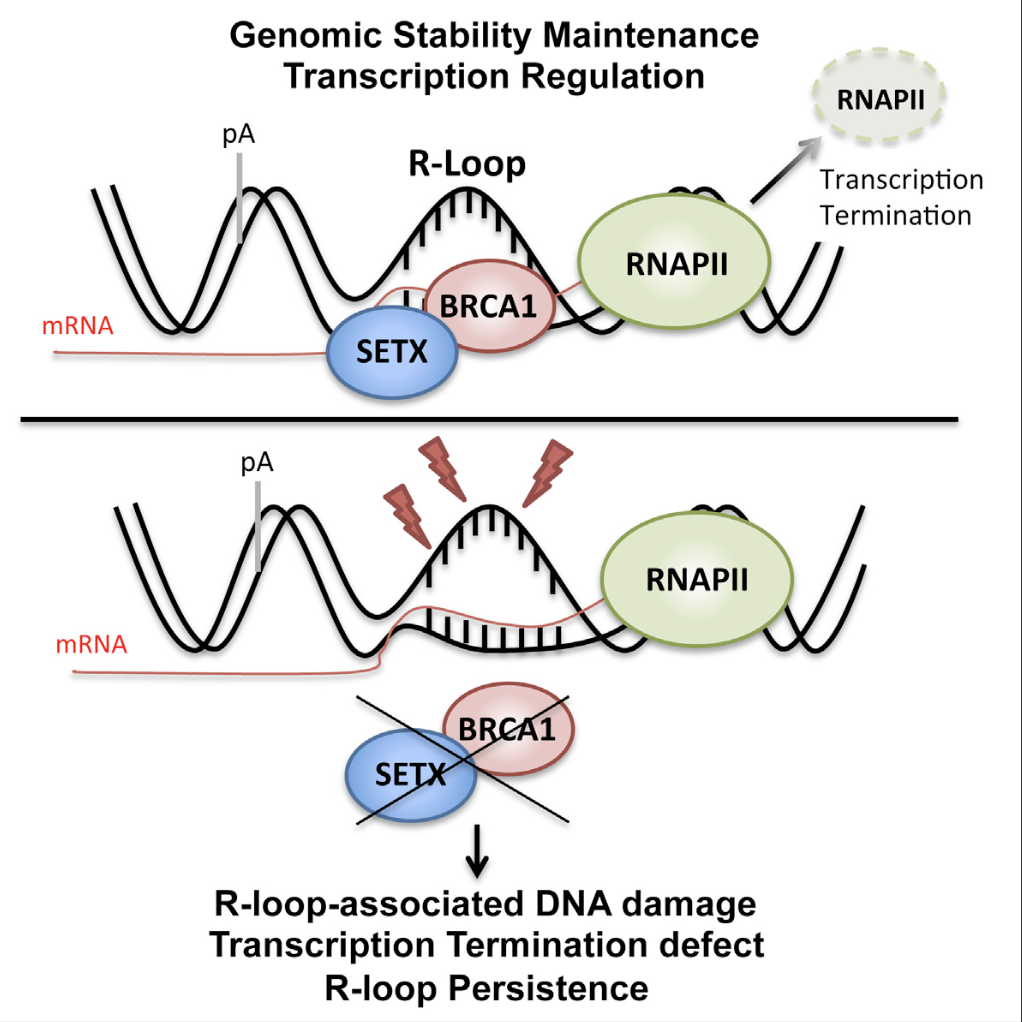
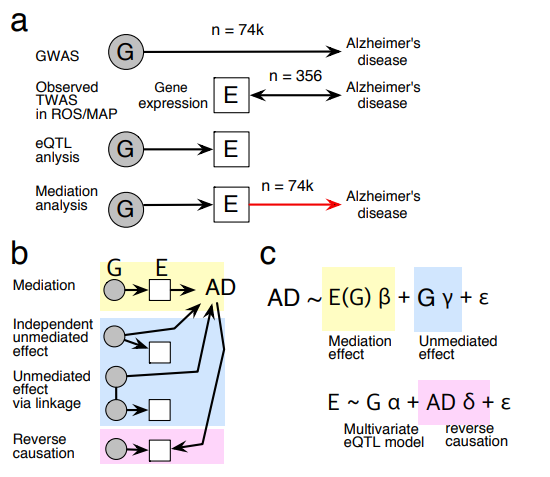
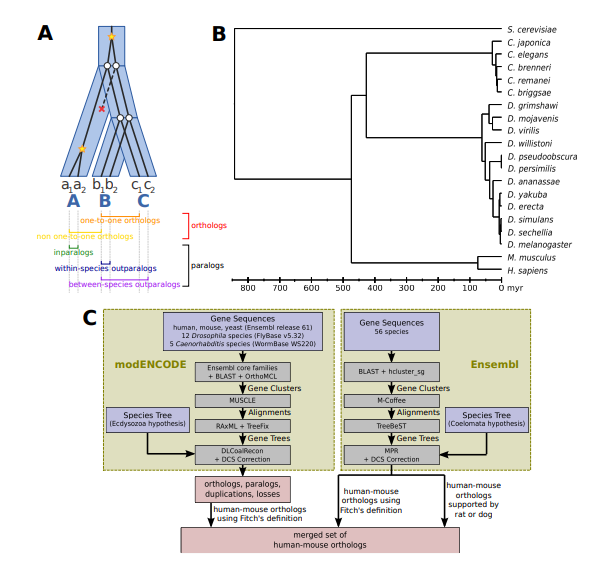
Jump To:
Preprints
Journal Publications
View Our Interactive Explore Page
Journal Publications
324. Relationship between sex biases in gene expression and sex biases in autism and Alzheimer's disease
Fass, Mulvey, Chase, Yang, Selmanovic, Chaturvedi, Tycksen, Weiss, Dougherty
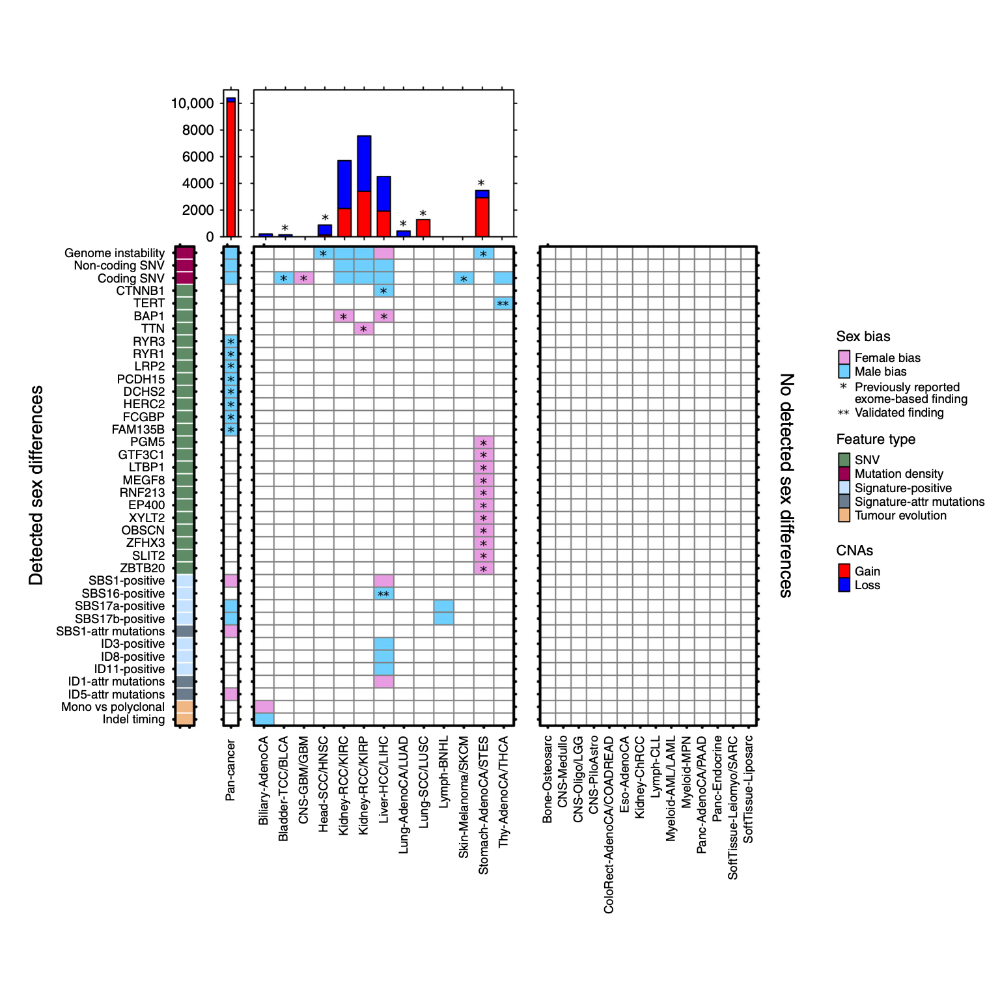
Sex differences in the brain may play an important role in sex-differential prevalence of neuropsychiatric conditions. In order to understand the transcriptional basis of sex differences, we analyzed multiple, large-scale, human postmortem brain RNA-Seq datasets using both within-region and pan-regional frameworks. We find evidence of sex-biased transcription in many autosomal genes, some of which provide evidence for pathways and cell population differences between chromosomally male and female individuals. These analyses also highlight regional differences in the extent of sex-differential gene expression. We observe an increase in specific neuronal transcripts in male brains and an increase in immune and glial function-related transcripts in female brains. Integration with single-nucleus data suggests this corresponds to sex differences in cellular states rather than cell abundance. Integration with case-control gene expression studies suggests a female molecular predisposition towards Alzheimer's disease, a female-biased disease. Autism, a male-biased diagnosis, does not exhibit a male predisposition pattern in our analysis. Overall, these analyses highlight mechanisms by which sex differences may interact with sex-biased conditions in the brain. Furthermore, we provide region-specific analyses of sex differences in brain gene expression to enable additional studies at the interface of gene expression and diagnostic differences.
Journal: Biology of Sex Differences, 2024 June 7 DOI: 10.1186/s13293-024-00622-2 PMID: 37693465.0 PMCID: PMC10491382
323. Single-cell multi-cohort dissection of the schizophrenia transcriptome
Ruzicka, Mohammadi, Fullard, Davila-Velderrain, Subburaju, Reed Tso, Hourihan, Jiang, Lee, Bendl, PsychENCODE Consortium, Voloudakis, Haroutunian, Hoffman, Roussos, Kellis
Schizophrenia population genomics has identified strong germline genetic associations for this highly heritable disorder, and molecular investigation of postmortem brain samples has yielded evidence of transcriptomic and epigenomic alterations associated with this disease. However, identifying molecular and cellular pathophysiological processes linking etiological risk factors and clinical presentation remains a challenge, due in part to the complex cellular architecture of the brain.
Journal: Science, 2024 May 24 DOI: 10.1126/science.adg5136 PMID: 38781388.0
322. Integrative multi-omics profiling in human decedents receiving pig heart xenografts
Schmauch, Piening, Mohebnasab, Xia, Zhu, Stern, Zhang, Dowdell, Kim, Andrijevic, Khalil, Jaffe, Loza, Gragert, Camellato, Oliveira, O’Brien, Chen, Weldon, Gao, Gandla, Chang, Bhatt, Gao, Lin, Reddy, Kagermazova, Habara, Widawsky, Liang, Sall, Loupy, Heguy, Taylor, Zhu, Michael, Jiang, Jian, Chong, Fairchild, Linna-Kuosmanen, Kaikkonen, Tatapudi, Lorber, Ayares, Mangiola, Narula, Moazami, Pass, Herati, Griesemer, Kellis, Snyder, Montgomery, Boeke, Keating
In a previous study, heart xenografts from 10-gene-edited pigs transplanted into two human decedents did not show evidence of acute-onset cellular- or antibody-mediated rejection. Here, to better understand the detailed molecular landscape following xenotransplantation, we carried out bulk and single-cell transcriptomics, lipidomics, proteomics and metabolomics on blood samples obtained from the transplanted decedents every 6 h, as well as histological and transcriptomic tissue profiling. We observed substantial early immune responses in peripheral blood mononuclear cells and xenograft tissue obtained from decedent 1 (male), associated with downstream T cell and natural killer cell activity. Longitudinal analyses indicated the presence of ischemia reperfusion injury, exacerbated by inadequate immunosuppression of T cells, consistent with previous findings of perioperative cardiac xenograft dysfunction in pig-to-nonhuman primate studies. Moreover, at 42 h after transplantation, substantial alterations in cellular metabolism and liver-damage pathways occurred, correlating with profound organ-wide physiological dysfunction. By contrast, relatively minor changes in RNA, protein, lipid and metabolism profiles were observed in decedent 2 (female) as compared to decedent 1. Overall, these multi-omics analyses delineate distinct responses to cardiac xenotransplantation in the two human decedents and reveal new insights into early molecular and immune responses after xenotransplantation. These findings may aid in the development of targeted therapeutic approaches to limit ischemia reperfusion injury-related phenotypes and improve outcomes.
Journal: Nature Medicine, 2024 May 17 DOI: 10.1038/s41591-024-02972-1 PMID: 38760586.0
321. TDP-43 proteinopathy in ALS is triggered by loss of ASRGL1 and associated with HML-2 expression
Garcia-Montojo, Fathi, Rastegar, Simula, Doucet-O'Hare, Cheng, Abrams, Pasternack, Malik, Bachani, Disanza, Maric, Lee, Wang, Santamaria, Li, Sampson, Lorenzo, Sanchez, Mezghrani, Li, Sechi, Pineda, Heiman, Kellis, Steiner, Nath
TAR DNA-binding protein 43 (TDP-43) proteinopathy in brain cells is the hallmark of amyotrophic lateral sclerosis (ALS) but its cause remains elusive. Asparaginase-like-1 protein (ASRGL1) cleaves isoaspartates, which alter protein folding and susceptibility to proteolysis. ASRGL1 gene harbors a copy of the human endogenous retrovirus HML-2, whose overexpression contributes to ALS pathogenesis. Here we show that ASRGL1 expression was diminished in ALS brain samples by RNA sequencing, immunohistochemistry, and western blotting. TDP-43 and ASRGL1 colocalized in neurons but, in the absence of ASRGL1, TDP-43 aggregated in the cytoplasm. TDP-43 was found to be prone to isoaspartate formation and a substrate for ASRGL1. ASRGL1 silencing triggered accumulation of misfolded, fragmented, phosphorylated and mislocalized TDP-43 in cultured neurons and motor cortex of female mice. Overexpression of ASRGL1 restored neuronal viability. Overexpression of HML-2 led to ASRGL1 silencing. Loss of ASRGL1 leading to TDP-43 aggregation may be a critical mechanism in ALS pathophysiology.
Journal: Nature Communications, 2024 May 16 DOI: 10.1038/s41467-024-48488-7 PMID: 38755145.0 PMCID: PMC11099023
320. A Polygenic Score Method Boosted by Non-additive Models
Ohta, Tanigawa, Suzuki, Kellis, Morishita
Dominance heritability in complex traits has received increasing recognition. However, most polygenic score (PGS) approaches do not incorporate non-additive effects. Here, we present GenoBoost, a flexible PGS modeling framework capable of considering both additive and non-additive effects, specifically focusing on genetic dominance. Building on statistical boosting theory, we derive provably optimal GenoBoost scores and provide its efficient implementation for analyzing large-scale cohorts. We benchmark it against seven commonly used PGS methods and demonstrate its competitive predictive performance. GenoBoost is ranked the best for four traits and second-best for three traits among twelve tested disease outcomes in UK Biobank. We reveal that GenoBoost improves prediction for autoimmune diseases by incorporating non-additive effects localized in the MHC locus and, more broadly, works best in less polygenic traits. We further demonstrate that GenoBoost can infer the mode of genetic inheritance without requiring prior knowledge. For example, GenoBoost finds non-zero genetic dominance effects for 602 of 900 selected genetic variants, resulting in 2.5% improvements in predicting psoriasis cases. Lastly, we show that GenoBoost can prioritize genetic loci with genetic dominance not previously reported in the GWAS catalog. Our results highlight the increased accuracy and biological insights from incorporating non-additive effects in PGS models.
Journal: Nature Communications, 2024 DOI: nan PMID: nan
319. Targeting IL-1 controls refractory Pityriasis rubra pilaris
Schmauch, Severin, Xing, Mangold, Conrad, Johannsen, Kahlenberg, Mellett, Navarini, Nobbe, Sarkar, Satyam, Tsoi, French, Linna-Kuosmanen, Kaikkonen, Snijder, Kellis, Gudjonsson, Tsokos, Contassot, Kolios
Pityriasis rubra pilaris (PRP) is a rare inflammatory skin disease which lacks efficacious standard-of-care treatments. Molecular studies of skin lesions revealed that IL-1Beta is central to the pathogenesis of PRP. Treatment of three patients with the IL-1-targeting biologics anakinra and canakinumab resulted in rapid clinical improvement and reversal of the PRP-associated molecular signature. We identified an NF-kappaB-mediated IL-1Beta-CCL20 axis central to the inflammatory response in PRP. Our results reveal the central role of IL-1Beta signaling in the pathogenesis of PRP and highlights its prominence as a therapeutic target.
Journal: nan, 2024 DOI: 10.21203/rs.3.rs-3433295/v1 PMID: nan
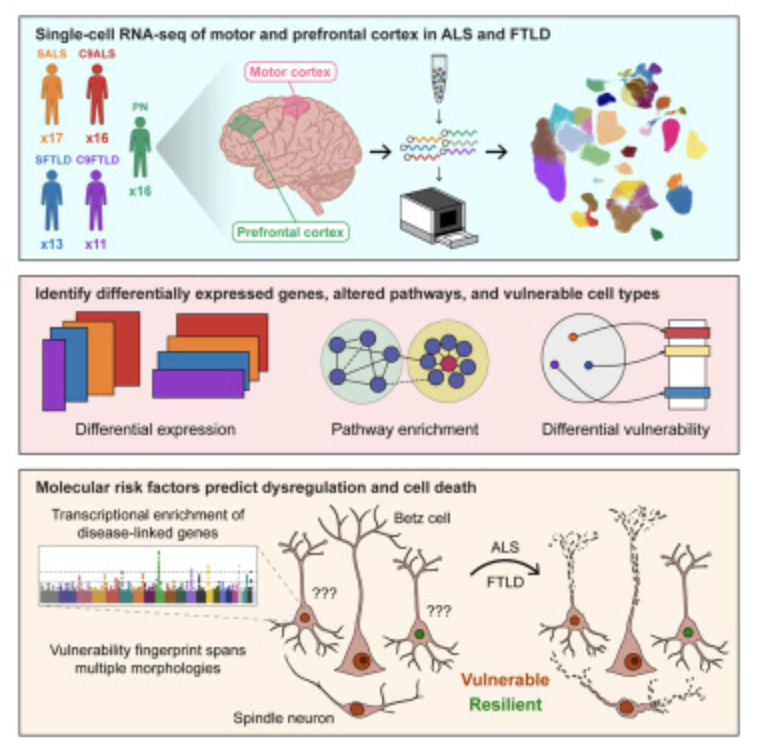
318. Single-cell dissection of the human motor and prefrontal cortices in ALS and FTLD
Pineda, Lee, Ulloa-Navas, Linville, Garcia, Galani, Engelberg-Cook, Castanedes, Fitzwalter, Pregent, Gardashli, DeTure, Vera-Garcia, Hucke, Oskarsson, Murray, Dickson, Heiman, Belzil, Kellis
Amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) share many clinical, pathological, and genetic features, but a detailed understanding of their associated transcriptional alterations across vulnerable cortical cell types is lacking. Here, we report a high-resolution, comparative single-cell molecular atlas of the human primary motor and dorsolateral prefrontal cortices and their transcriptional alterations in sporadic and familial ALS and FTLD. By integrating transcriptional and genetic information, we identify known and previously unidentified vulnerable populations in cortical layer 5 and show that ALS- and FTLD-implicated motor and spindle neurons possess a virtually indistinguishable molecular identity. We implicate potential disease mechanisms affecting these cell types as well as non-neuronal drivers of pathogenesis. Finally, we show that neuron loss in cortical layer 5 tracks more closely with transcriptional identity rather than cellular morphology and extends beyond previously reported vulnerable cell types.
Journal: Cell, 2024 Mar 22 DOI: 10.1016/j.cell.2024.02.031 PMID: 38521060.0
317. Gamma entrainment using audiovisual stimuli alleviates chemobrain pathology and cognitive impairment induced by chemotherapy in mice
Kim, James, Kahn, Blanco-Duque, Abdurrob, Islam, Lavoie, Kellis, Tsai
Patients with cancer undergoing chemotherapy frequently experience a neurological condition known as chemotherapy-related cognitive impairment, or "chemobrain," which can persist for the remainder of their lives. Despite the growing prevalence of chemobrain, both its underlying mechanisms and treatment strategies remain poorly understood. Recent findings suggest that chemobrain shares several characteristics with neurodegenerative diseases, including chronic neuroinflammation, DNA damage, and synaptic loss. We investigated whether a noninvasive sensory stimulation treatment we term gamma entrainment using sensory stimuli (GENUS), which has been shown to alleviate aberrant immune and synaptic pathologies in mouse models of neurodegeneration, could also mitigate chemobrain phenotypes in mice administered a chemotherapeutic drug. When administered concurrently with the chemotherapeutic agent cisplatin, GENUS alleviated cisplatin-induced brain pathology, promoted oligodendrocyte survival, and improved cognitive function in a mouse model of chemobrain. These effects persisted for up to 105 days after GENUS treatment, suggesting the potential for long-lasting benefits. However, when administered to mice 90 days after chemotherapy, GENUS treatment only provided limited benefits, indicating that it was most effective when used to prevent the progression of chemobrain pathology. Furthermore, we demonstrated that the effects of GENUS in mice were not limited to cisplatin-induced chemobrain but also extended to methotrexate-induced chemobrain. Collectively, these findings suggest that GENUS may represent a versatile approach for treating chemobrain induced by different chemotherapy agents.
Journal: Science Translational Medicine, 2024 Mar 6 DOI: 10.1126/scitranslmed.adf4601 PMID: 38446899.0
316. New approaches for understanding the potential role of microbes in Alzheimer's disease
Whitson, Banks, Diaz, Frost, Kellis, Lathe, Schmader, Spudich, Tanzi, Garden
Alzheimer's disease (AD) involves a complex pathological process that evolves over years, and its etiology is understood as a classic example of gene-environment interaction. The notion that exposure to microbial organisms may play some role in AD pathology has been proposed and debated for decades. New evidence from model organisms and -omic studies, as well as epidemiological data from the recent COVID-19 pandemic and widespread use of vaccines, offers new insights into the "germ hypothesis" of AD. To review new evidence and identify key research questions, the Duke/University of North Carolina (Duke/UNC) Alzheimer's Disease Research Center hosted a virtual symposium and workshop: "New Approaches for Understanding the Potential Role of Microbes in Alzheimer's disease." Discussion centered around the antimicrobial protection hypothesis of amyloid accumulation, and other mechanisms by which microbes could influence AD pathology including immune cell activation, changes in blood-brain barrier, or direct neurotoxicity. This summary of proceedings reviews the content presented in the symposium and provides a summary of major topics and key questions discussed in the workshop.
Journal: Brain Behavior, and Immunity - Health, 2024 Feb 21 DOI: 10.1016/j.bbih.2024.100743 PMID: 38435720.0 PMCID: PMC10906156
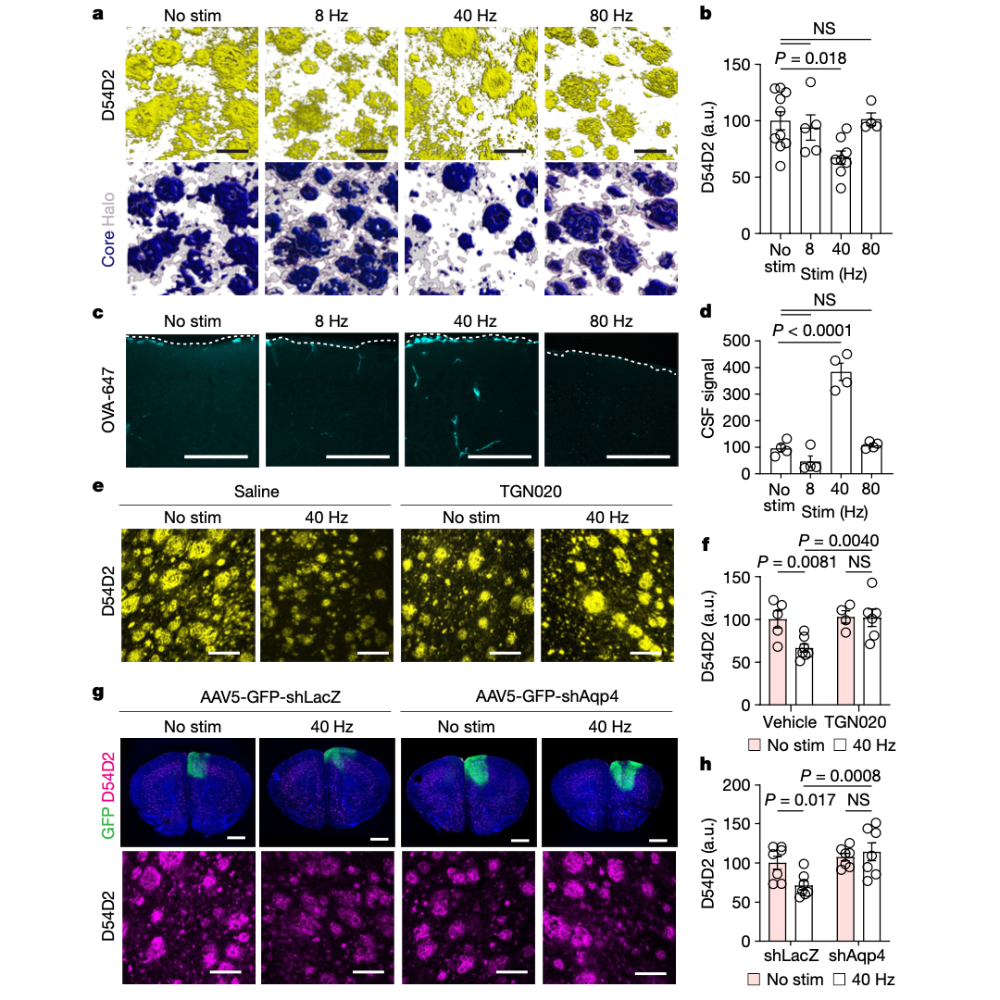
315. Multisensory gamma stimulation promotes glymphatic clearance of amyloid
Murdock, Yang, Sun, Pao, Blanco-Duque, Kahn, Kim, Lavoie, Victor, Islam, Galiana, Leary, Wang, Bubnys, Ma, Akay, Sneve, Qian, Lai, McCarthy, Kopell, Kellis, Piatkevich, Boyden, Tsai
The glymphatic movement of fluid through the brain removes metabolic waste1-4. Noninvasive 40 Hz stimulation promotes 40 Hz neural activity in multiple brain regions and attenuates pathology in mouse models of Alzheimer's disease5-8. Here we show that multisensory gamma stimulation promotes the influx of cerebrospinal fluid and the efflux of interstitial fluid in the cortex of the 5XFAD mouse model of Alzheimer's disease. Influx of cerebrospinal fluid was associated with increased aquaporin-4 polarization along astrocytic endfeet and dilated meningeal lymphatic vessels. Inhibiting glymphatic clearance abolished the removal of amyloid by multisensory 40 Hz stimulation. Using chemogenetic manipulation and a genetically encoded sensor for neuropeptide signalling, we found that vasoactive intestinal peptide interneurons facilitate glymphatic clearance by regulating arterial pulsatility. Our findings establish novel mechanisms that recruit the glymphatic system to remove brain amyloid.
Journal: Nature Communications, 2024 Feb 28 DOI: 10.1038/s41586-024-07132-6 PMID: 38418876.0 PMCID: PMC10917684
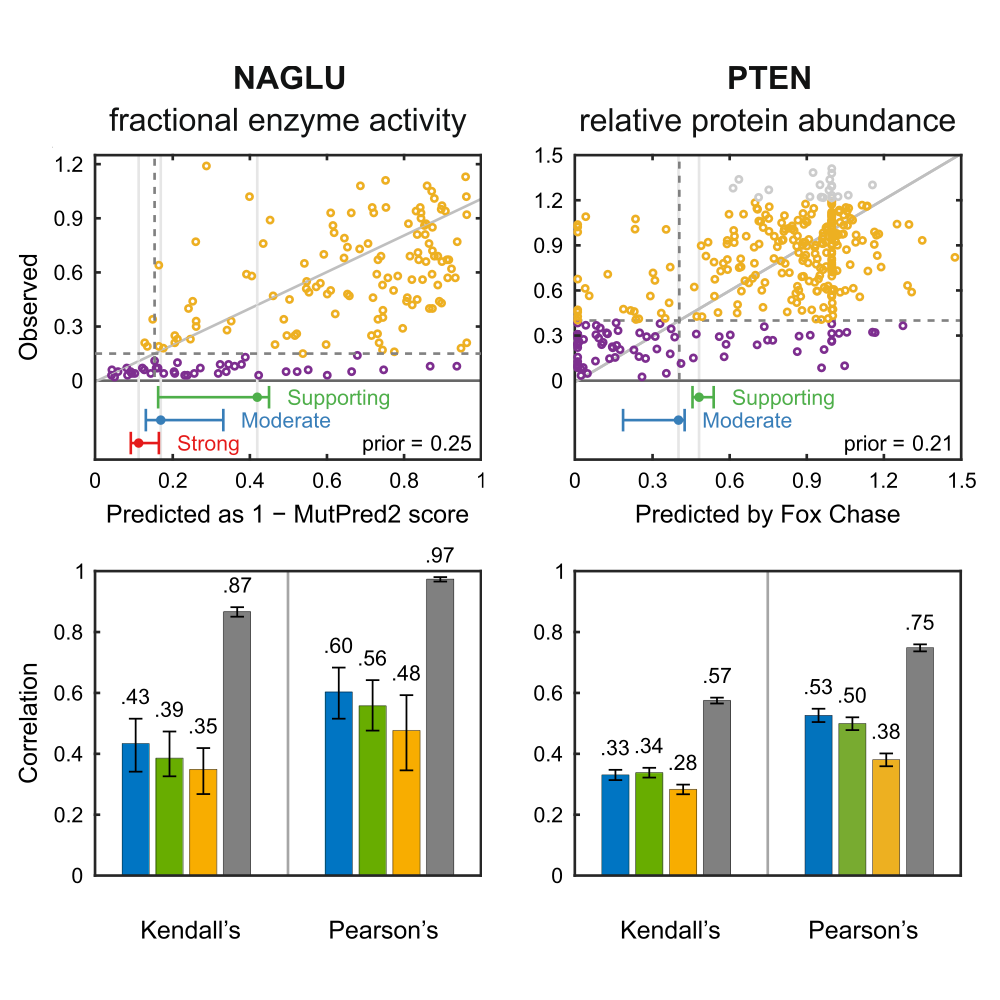
314. CAGI, the Critical Assessment of Genome Interpretation, establishes progress and prospects for computational genetic variant interpretation methods
Critical Assessment of Genome Interpretation Consortium
The Critical Assessment of Genome Interpretation (CAGI) aims to advance the state-of-the-art for computational prediction of genetic variant impact, particularly where relevant to disease. The five complete editions of the CAGI community experiment comprised 50 challenges, in which participants made blind predictions of phenotypes from genetic data, and these were evaluated by independent assessors. Performance was particularly strong for clinical pathogenic variants, including some difficult-to-diagnose cases, and extends to interpretation of cancer-related variants. Missense variant interpretation methods were able to estimate biochemical effects with increasing accuracy. Assessment of methods for regulatory variants and complex trait disease risk was less definitive and indicates performance potentially suitable for auxiliary use in the clinic. Results show that while current methods are imperfect, they have major utility for research and clinical applications. Emerging methods and increasingly large, robust datasets for training and assessment promise further progress ahead.
Journal: Genome Biology, 2024 Feb 22 DOI: 10.1186/s13059-023-03113-6 PMID: 38389099.0 PMCID: PMC10882881
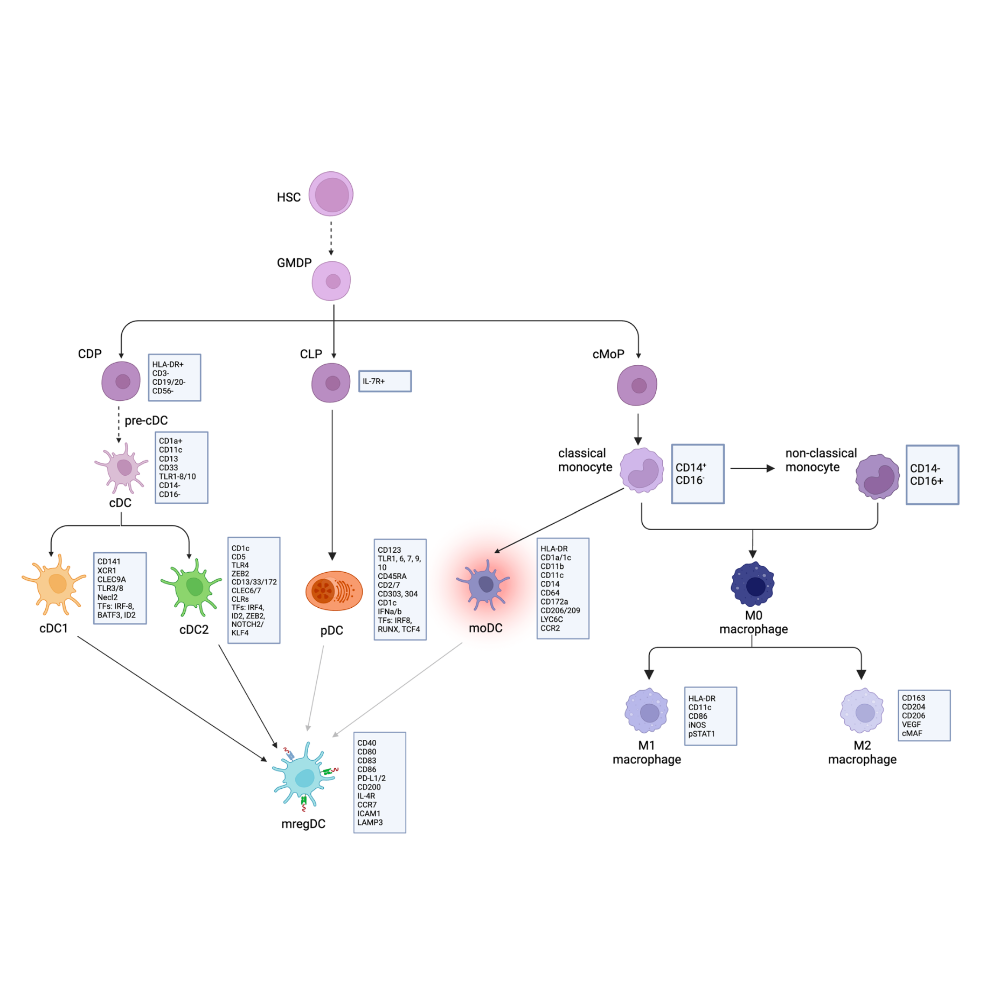
313. Antigen presenting cells in cancer immunity and mediation of immune checkpoint blockade
Wang, Chen, Fu, Liu, Puri, Kellis, Yang
Antigen-presenting cells (APCs) are pivotal mediators of immune responses. Their role has increasingly been spotlighted in the realm of cancer immunology, particularly as our understanding of immunotherapy continues to evolve and improve. There is growing evidence that these cells play a non-trivial role in cancer immunity and have roles dependent on surface markers, growth factors, transcription factors, and their surrounding environment. The main dendritic cell (DC) subsets found in cancer are conventional DCs (cDC1 and cDC2), monocyte-derived DCs (moDC), plasmacytoid DCs (pDC), and mature and regulatory DCs (mregDC). The notable subsets of monocytes and macrophages include classical and non-classical monocytes, macrophages, which demonstrate a continuum from a pro-inflammatory (M1) phenotype to an anti-inflammatory (M2) phenotype, and tumor-associated macrophages (TAMs). Despite their classification in the same cell type, each subset may take on an immune-activating or immunosuppressive phenotype, shaped by factors in the tumor microenvironment (TME). In this review, we introduce the role of DCs, monocytes, and macrophages and recent studies investigating them in the cancer immunity context. Additionally, we review how certain characteristics such as abundance, surface markers, and indirect or direct signaling pathways of DCs and macrophages may influence tumor response to immune checkpoint blockade (ICB) therapy. We also highlight existing knowledge gaps regarding the precise contributions of different myeloid cell subsets in influencing the response to ICB therapy. These findings provide a summary of our current understanding of myeloid cells in mediating cancer immunity and ICB and offer insight into alternative or combination therapies that may enhance the success of ICB in cancers.
Journal: Clinical & Experimental Metastasis, 2024 Jan 23 DOI: 10.1007/s10585-023-10257-z PMID: 38261139.0
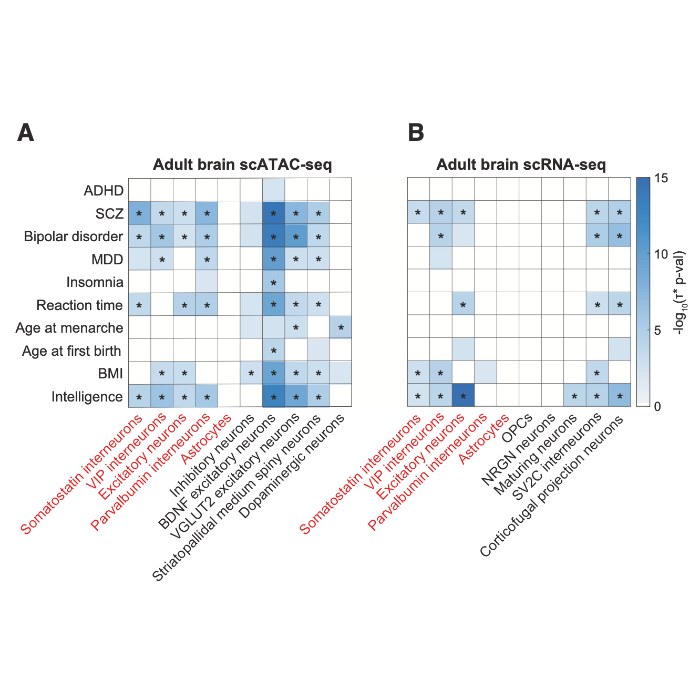
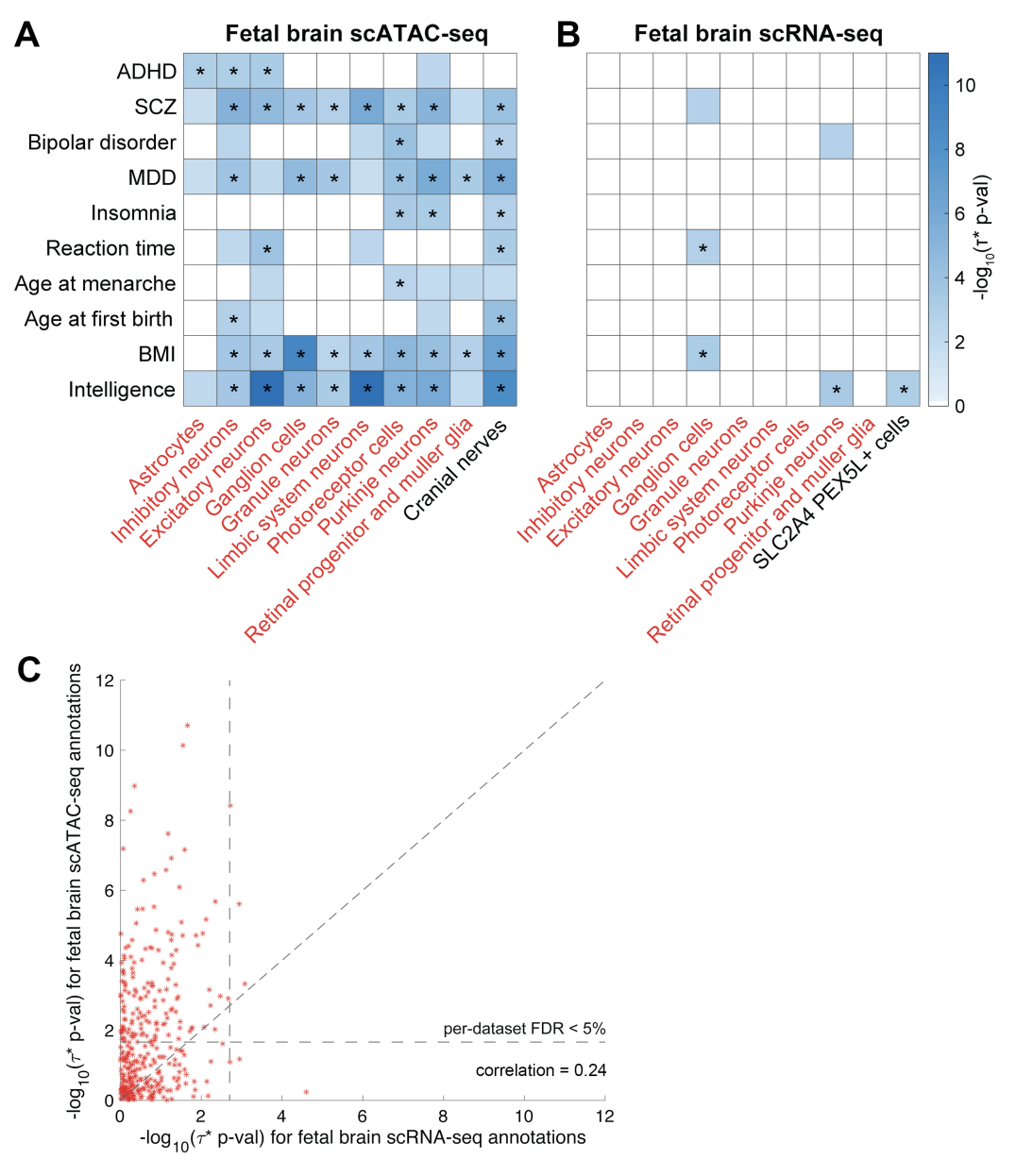
312. Leveraging single-cell ATAC-seq and RNA-seq to identify disease-critical fetal and adult brain cell types
Kim, Truong, Jagadeesh, Dey, Shen, Raychaudhuri, Kellis, Price
Prioritizing disease-critical cell types by integrating genome-wide association studies (GWAS) with functional data is a fundamental goal. Single-cell chromatin accessibility (scATAC-seq) and gene expression (scRNA-seq) have characterized cell types at high resolution, and studies integrating GWAS with scRNA-seq have shown promise, but studies integrating GWAS with scATAC-seq have been limited. Here, we identify disease-critical fetal and adult brain cell types by integrating GWAS summary statistics from 28 brain-related diseases/traits (average N = 298 K) with 3.2 million scATAC-seq and scRNA-seq profiles from 83 cell types. We identified disease-critical fetal (respectively adult) brain cell types for 22 (respectively 23) of 28 traits using scATAC-seq, and for 8 (respectively 17) of 28 traits using scRNA-seq. Significant scATAC-seq enrichments included fetal photoreceptor cells for major depressive disorder, fetal ganglion cells for BMI, fetal astrocytes for ADHD, and adult VGLUT2 excitatory neurons for schizophrenia. Our findings improve our understanding of brain-related diseases/traits and inform future analyses.
Journal: Nature Communications, 2024 Jan 17 DOI: 10.1038/s41467-024-44742-0 PMID: 38233398.0
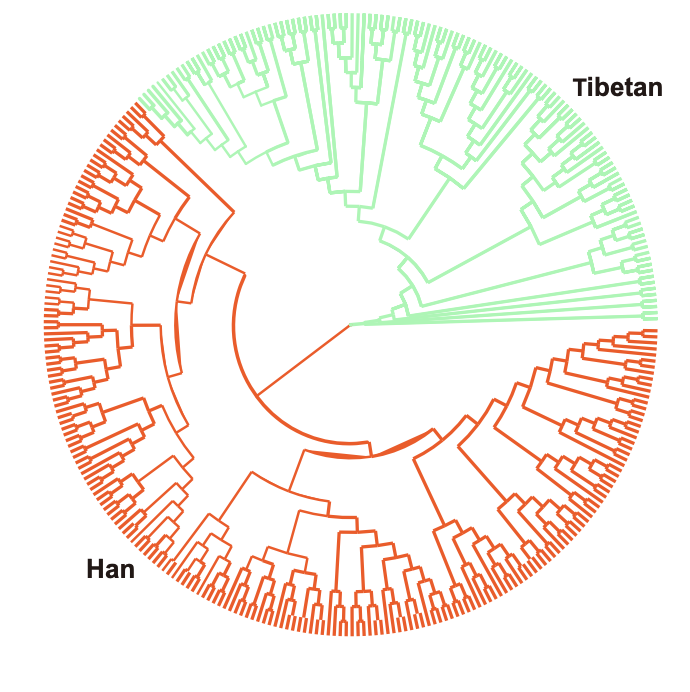
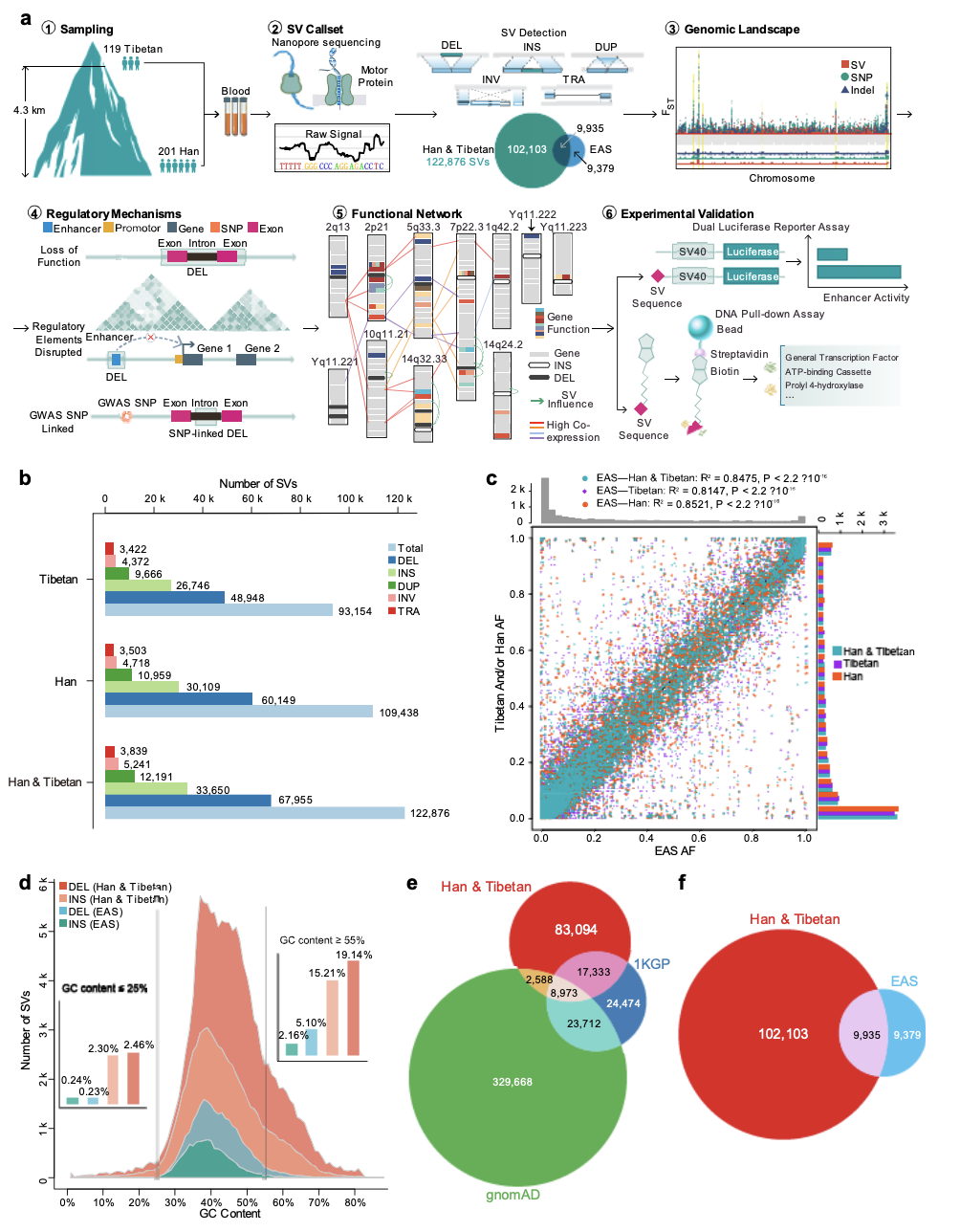
311. Structural variants involved in high-altitude adaptation detected using single-molecule long-read sequencing.
Shi, Jia, Sun, Wang, Zhao, Zhao, Liang, Song, Guan, Jia, Yang, Chen, Yu, Jia, Wu, Wang, Xiao, Xu, Liu, Wu, Zhong, Wu, Cui, Bo, Wu, Park, Kellis, He
Structural variants (SVs), accounting for a larger fraction of the genome than SNPs/InDels, are an important pool of genetic variation, enabling environmental adaptations. Here, we perform long-read sequencing data of 320 Tibetan and Han samples and show that SVs are highly involved in high-altitude adaptation. We expand the landscape of global SVs, apply robust models of selection and population differentiation combining SVs, SNPs and InDels, and use epigenomic analyses to predict enhancers, target genes and biological functions. We reveal diverse Tibetan-specific SVs affecting the regulatory circuitry of biological functions, including the hypoxia response, energy metabolism and pulmonary function. We find a Tibetan-specific deletion disrupts a super-enhancer and downregulates EPAS1 using enhancer reporter, cellular knock-out and DNA pull-down assays. Our study expands the global SV landscape, reveals the role of gene-regulatory circuitry rewiring in human adaptation, and illustrates the diverse functional roles of SVs in human biology.
Journal: Nature Communications, 2023 Dec 13 DOI: 10.1038/s41467-023-44034-z PMID: 38092772.0 PMCID: PMC10719358
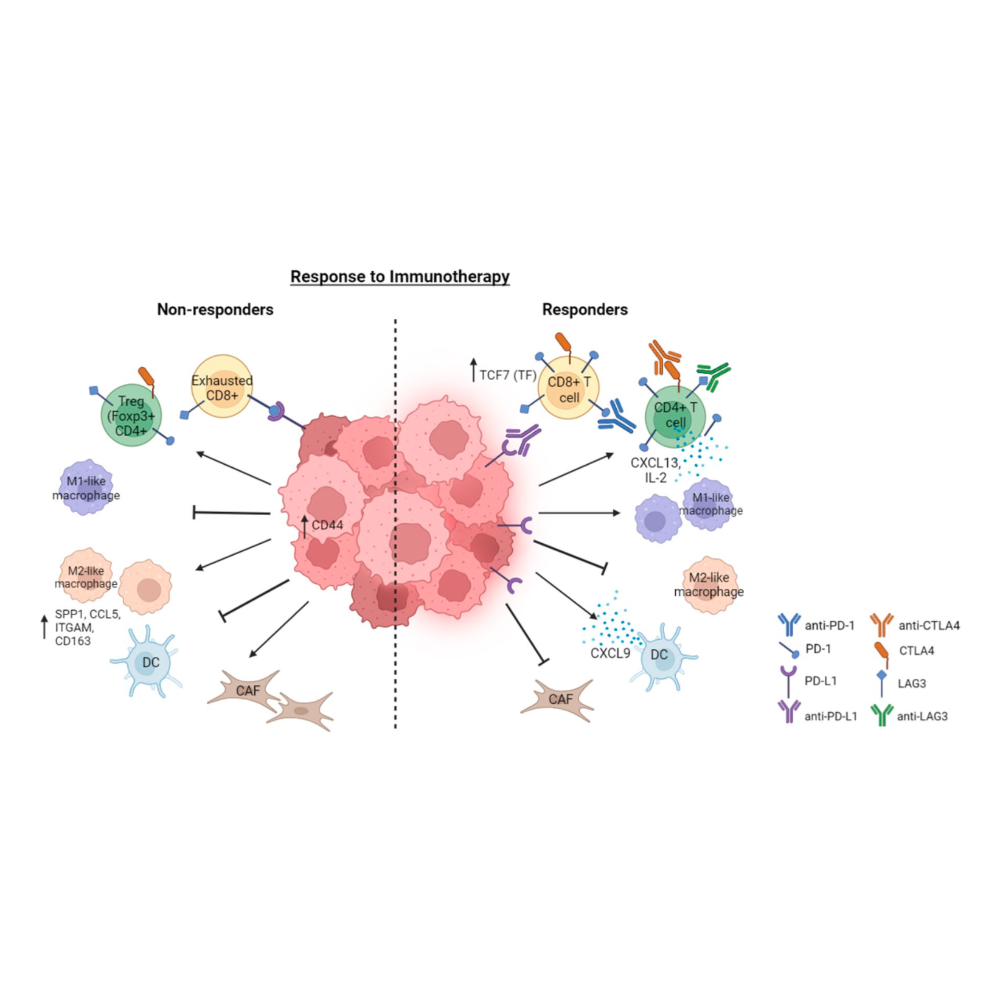
310. Dissecting the tumor microenvironment in response to immune checkpoint inhibitors via single-cell and spatial transcriptomics.
Liu, Puri, Fu, Chen, Wang, Kellis, Yang
Cancer is a disease that undergoes selective pressure to evolve during its progression, becoming increasingly heterogeneous. Tumoral heterogeneity can dictate therapeutic response. Transcriptomics can be used to uncover complexities in cancer and reveal phenotypic heterogeneity that affects disease response. This is especially pertinent in the immune microenvironment, which contains diverse populations of immune cells, and whose dynamic properties influence disease response. The recent development of immunotherapies has revolutionized cancer therapy, with response rates of up to 50% within certain cancers. However, despite advances in immune checkpoint blockade specifically, there remains a significant population of non-responders to these treatments. Transcriptomics can be used to profile immune and other cell populations following immune-checkpoint inhibitor (ICI) treatment, generate predictive biomarkers of resistance or response, assess immune effector function, and identify potential immune checkpoints. Single-cell RNA sequencing has offered insight into mRNA expression within the complex and heterogeneous tumor microenvironment at single-cell resolution. Spatial transcriptomics has enabled measurement of mRNA expression while adding locational context. Here, we review single-cell sequencing and spatial transcriptomic research investigating ICI response within a variety of cancer microenvironments.
Journal: Clinical & Experimental Metastasis, 2023 Dec 8 DOI: 10.1007/s10585-023-10246-2 PMID: 38064127.0
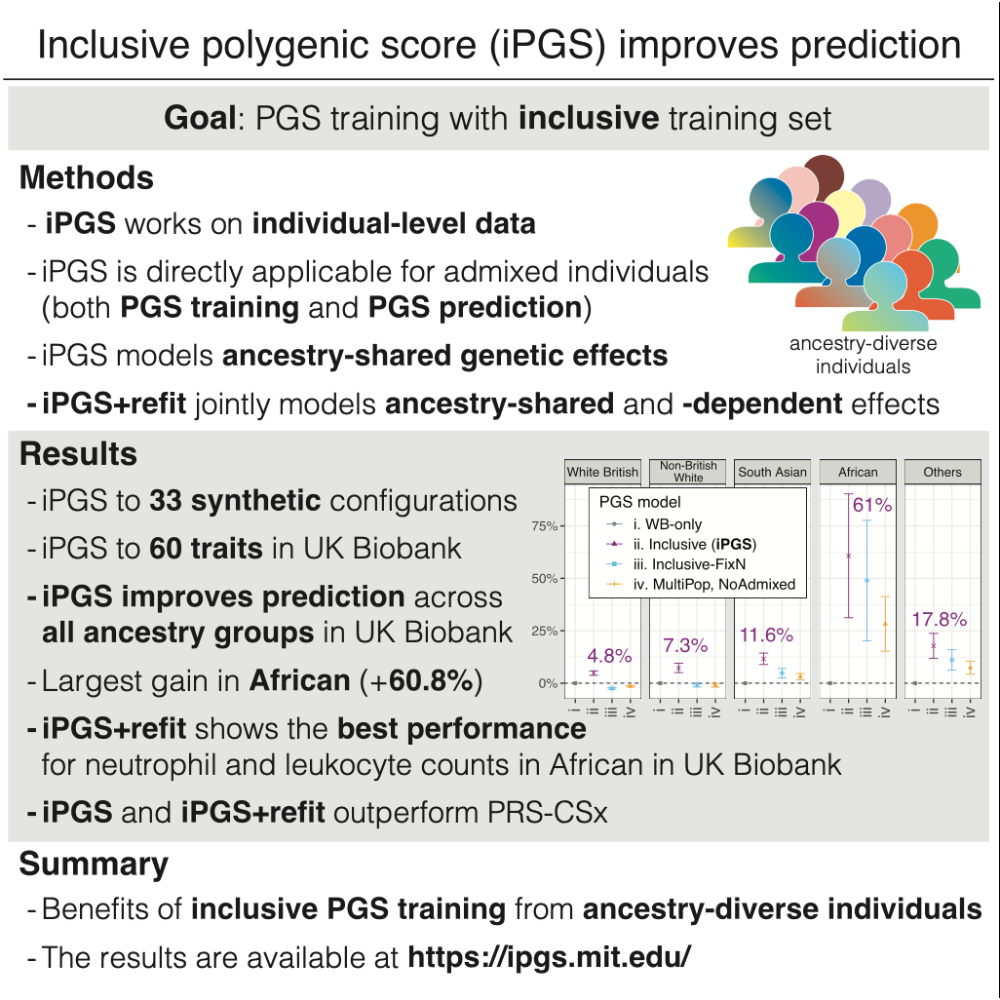
309. Power of inclusion: Enhancing polygenic prediction with admixed individuals.
Tanigawa, Kellis
Admixed individuals offer unique opportunities for addressing limited transferability in polygenic scores (PGSs), given the substantial trans-ancestry genetic correlation in many complex traits. However, they are rarely considered in PGS training, given the challenges in representing ancestry-matched linkage-disequilibrium reference panels for admixed individuals. Here we present inclusive PGS (iPGS), which captures ancestry-shared genetic effects by finding the exact solution for penalized regression on individual-level data and is thus naturally applicable to admixed individuals. We validate our approach in a simulation study across 33 configurations with varying heritability, polygenicity, and ancestry composition in the training set. When iPGS is applied to n = 237,055 ancestry-diverse individuals in the UK Biobank, it shows the greatest improvements in Africans by 48.9% on average across 60 quantitative traits and up to 50-fold improvements for some traits (neutrophil count, R(2) = 0.058) over the baseline model trained on the same number of European individuals. When we allowed iPGS to use n = 284,661 individuals, we observed an average improvement of 60.8% for African, 11.6% for South Asian, 7.3% for non-British White, 4.8% for White British, and 17.8% for the other individuals. We further developed iPGS+refit to jointly model the ancestry-shared and -dependent genetic effects when heterogeneous genetic associations were present. For neutrophil count, for example, iPGS+refit showed the highest predictive performance in the African group (R(2) = 0.115), which exceeds the best predictive performance for the White British group (R(2) = 0.090 in the iPGS model), even though only 1.49% of individuals used in the iPGS training are of African ancestry. Our results indicate the power of including diverse individuals for developing more equitable PGS models.
Journal: American Journal Of Human Genetics, 2023 Nov 2 DOI: 10.1016/j.ajhg.2023.09.013 PMID: 37890495.0 PMCID: PMC10645553
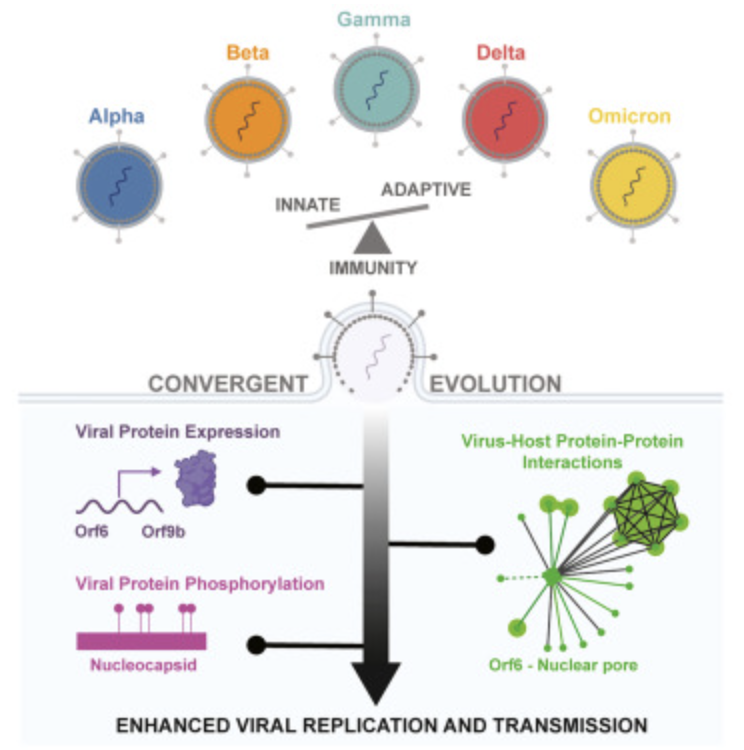
308. SARS-CoV-2 variants evolve convergent strategies to remodel the host response.
Bouhaddou, Reuschl, Polacco, Thorne, Ummadi, Ye, Rosales, Pelin, Batra, Jang, Xu, Moen, Richards, Zhou, Harjai, Stevenson, Rojc, Ragazzini, Whelan, Furnon, De, Cowton, Syed, Ciling, Deutsch, Pirak, Dowgier, Mesner, Turner, McGovern, Rodriguez, Leiva-Rebollo, Dunham, Zhong, Eckhardt, Fossati, Liotta, Kehrer, Cupic, Rutkowska, Mena, Aslam, Hoffert, Foussard, Olwal, Huang, Zwaka, Pham, Lyons, Donohue, Griffin, Nugent, Holden, Deans, Aviles, Lopez-Martin, Jimeno, Obernier, Fabius, Soucheray, Huttenhain, Jungreis, Kellis, Echeverria, Verba, Bonfanti, Beltrao, Sharan, Doudna, Martinez-Sobrido, Patel, Palmarini, Miorin, White, Swaney, Garcia-Sastre, Jolly, Zuliani-Alvarez, Towers, Krogan
SARS-CoV-2 variants of concern (VOCs) emerged during the COVID-19 pandemic. Here, we used unbiased systems approaches to study the host-selective forces driving VOC evolution. We discovered that VOCs evolved convergent strategies to remodel the host by modulating viral RNA and protein levels, altering viral and host protein phosphorylation, and rewiring virus-host protein-protein interactions. Integrative computational analyses revealed that although Alpha, Beta, Gamma, and Delta ultimately converged to suppress interferon-stimulated genes (ISGs), Omicron BA.1 did not. ISG suppression correlated with the expression of viral innate immune antagonist proteins, including Orf6, N, and Orf9b, which we mapped to specific mutations. Later Omicron subvariants BA.4 and BA.5 more potently suppressed innate immunity than early subvariant BA.1, which correlated with Orf6 levels, although muted in BA.4 by a mutation that disrupts the Orf6-nuclear pore interaction. Our findings suggest that SARS-CoV-2 convergent evolution overcame human adaptive and innate immune barriers, laying the groundwork to tackle future pandemics.
Journal: Cell, 2023 Oct 12 DOI: 10.1016/j.cell.2023.08.026 PMID: 37738970.0 PMCID: PMC10604369
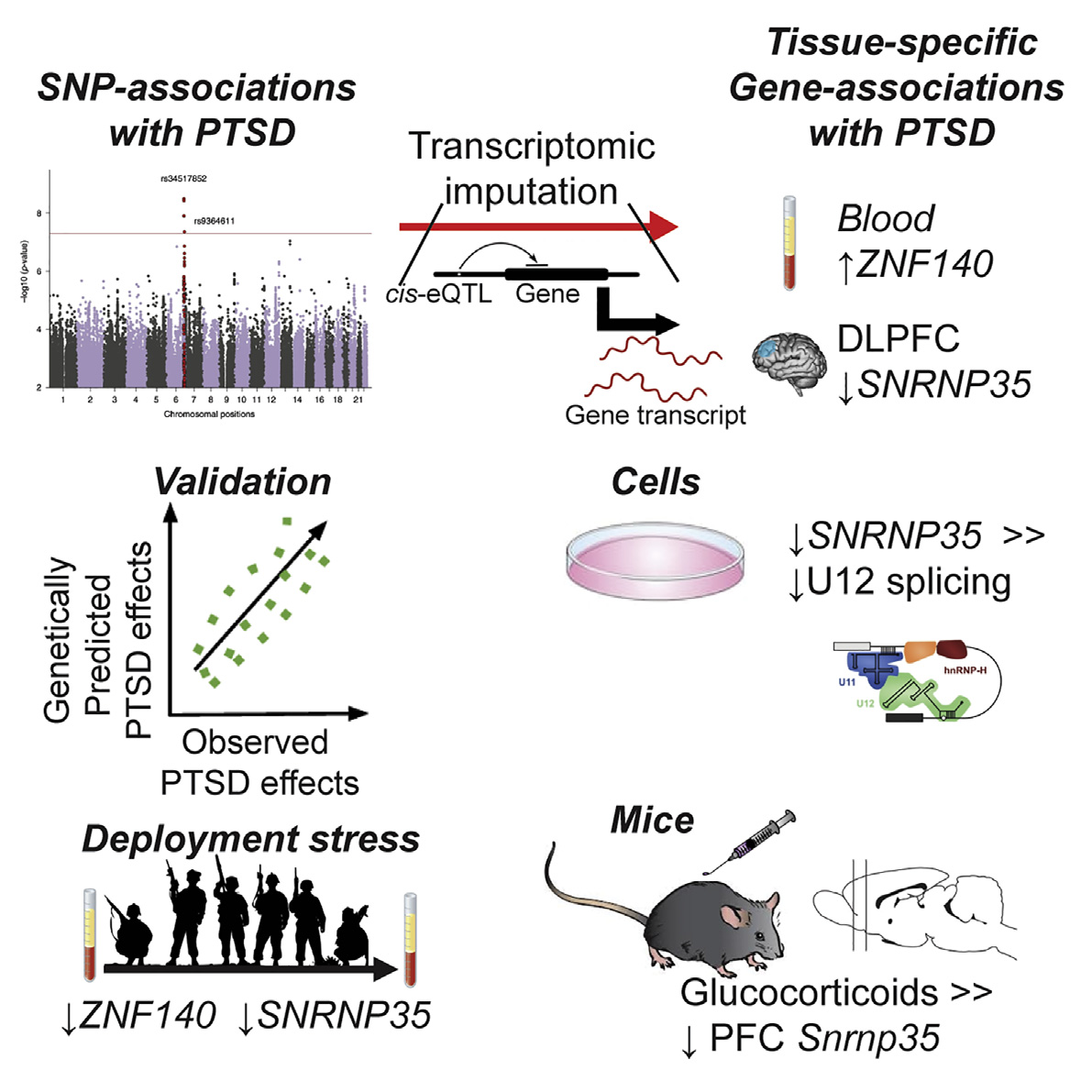
307. Single-Nucleus Transcriptome Profiling of Dorsolateral Prefrontal Cortex: Mechanistic Roles for Neuronal Gene Expression, Including the 17q21.31 Locus, in PTSD Stress Response.
Chatzinakos, Pernia, Morrison, Iatrou, McCullough, Schuler, Snijders, Bajaj, DiPietro, Soliva, Gassen, Anastasopoulos, Bharadwaj, Bowlby, Hartmann, Maihofer, Nievergelt, Ressler, Wolf, Carlezon, Krystal, Kleinman, Girgenti, Huber, Kellis, Logue, Miller, Ressler, Daskalakis
Multidisciplinary studies of posttraumatic stress disorder (PTSD) and major depressive disorder (MDD) implicate the dorsolateral prefrontal cortex (DLPFC) in disease risk and pathophysiology. Postmortem brain studies have relied on bulk-tissue RNA sequencing (RNA-seq), but single-cell RNA-seq is needed to dissect cell-type-specific mechanisms. The authors conducted the first single-nucleus RNA-seq postmortem brain study in PTSD to elucidate disease transcriptomic pathology with cell-type-specific resolution. METHOD: Profiling of 32 DLPFC samples from 11 individuals with PTSD, 10 with MDD, and 11 control subjects was conducted ( approximately 415K nuclei; >13K cells per sample). A replication sample included 15 DLPFC samples ( approximately 160K nuclei; >11K cells per sample). RESULTS: Differential gene expression analyses identified significant single-nucleus RNA-seq differentially expressed genes (snDEGs) in excitatory (EX) and inhibitory (IN) neurons and astrocytes, but not in other cell types or bulk tissue. MDD samples had more false discovery rate-corrected significant snDEGs, and PTSD samples had a greater replication rate. In EX and IN neurons, biological pathways that were differentially enriched in PTSD compared with MDD included glucocorticoid signaling. Furthermore, glucocorticoid signaling in induced pluripotent stem cell (iPSC)-derived cortical neurons demonstrated greater relevance in PTSD and opposite direction of regulation compared with MDD, especially in EX neurons. Many snDEGs were from the 17q21.31 locus and are particularly interesting given causal roles in disease pathogenesis and DLPFC-based neuroimaging (PTSD: ARL17B, LINC02210-CRHR1, and LRRC37A2; MDD: LRRC37A and LRP4), while others were regulated by glucocorticoids in iPSC-derived neurons (PTSD: SLC16A6, TAF1C; MDD: CDH3). CONCLUSIONS: The study findings point to cell-type-specific mechanisms of brain stress response in PTSD and MDD, highlighting the importance of examining cell-type-specific gene expression and indicating promising novel biomarkers and therapeutic targets.
Journal: The American Journal Of Psychiatry, 2023 Oct 1 DOI: 10.1176/appi.ajp.20220478 PMID: 37491937.0
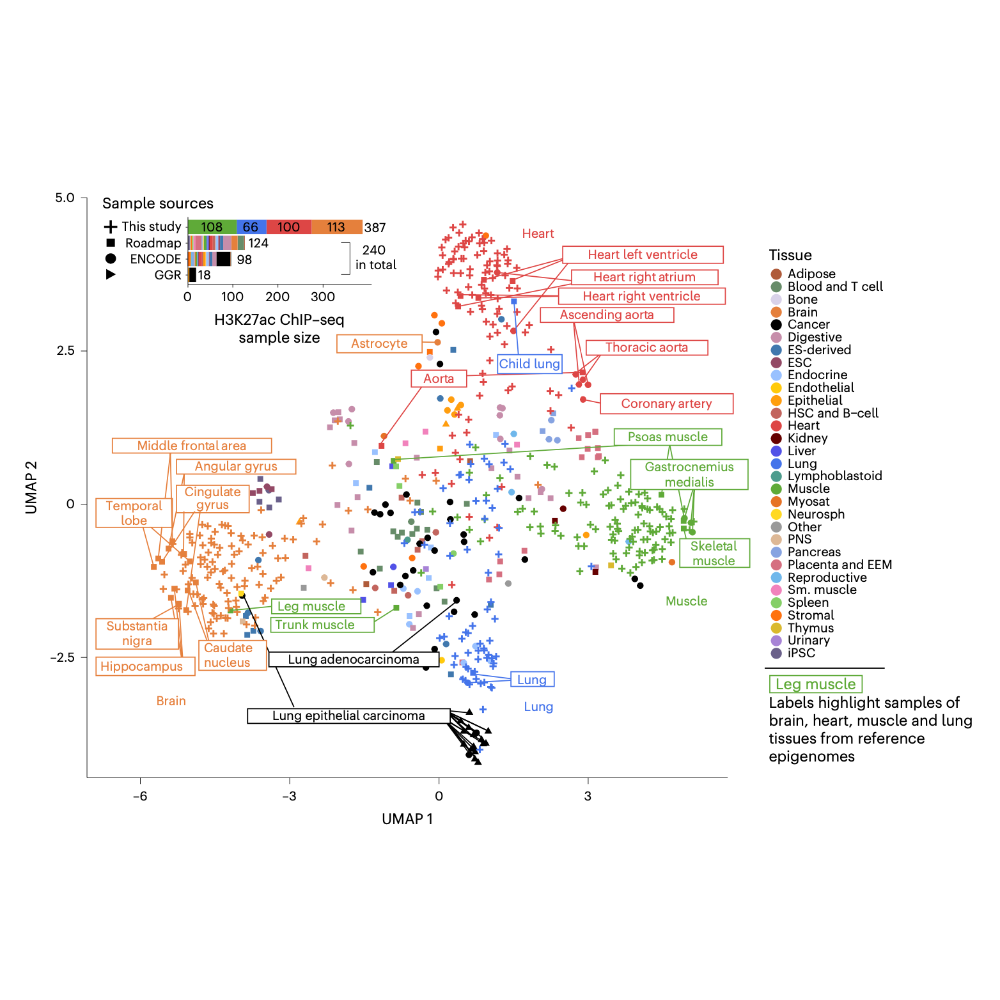
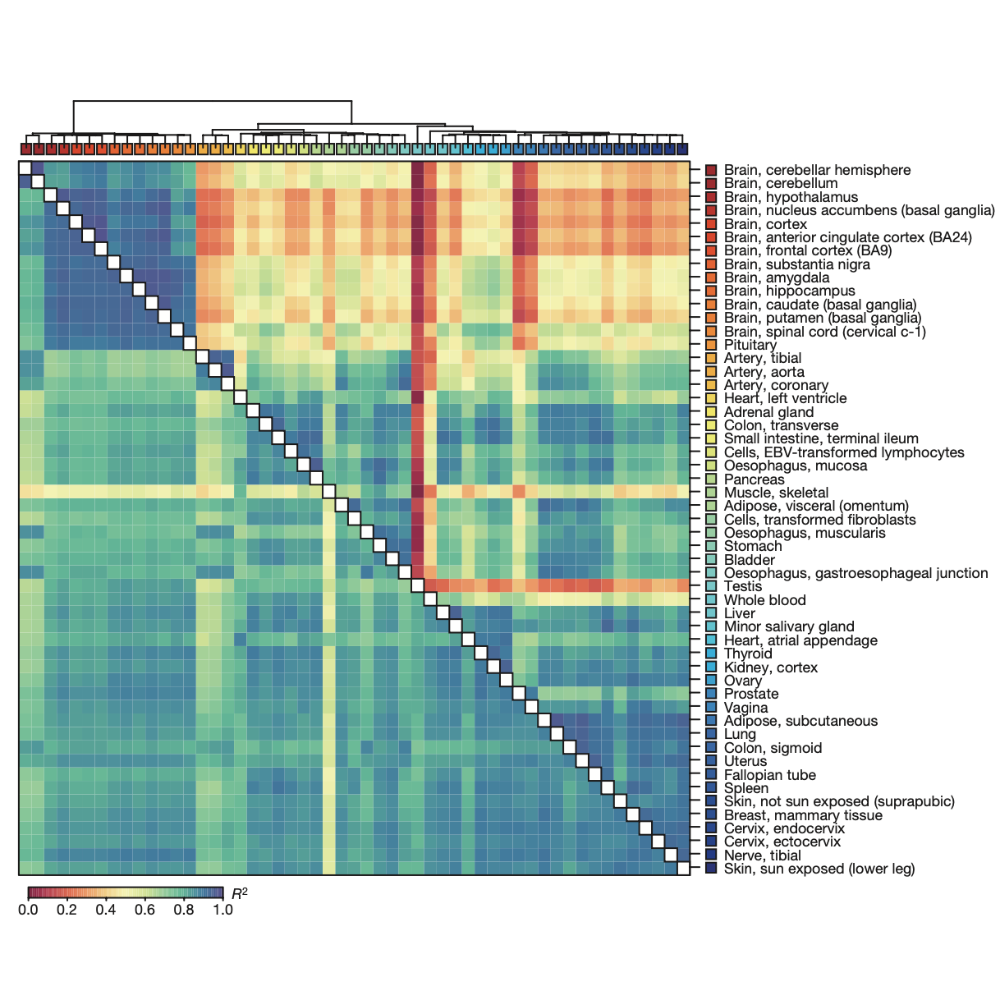
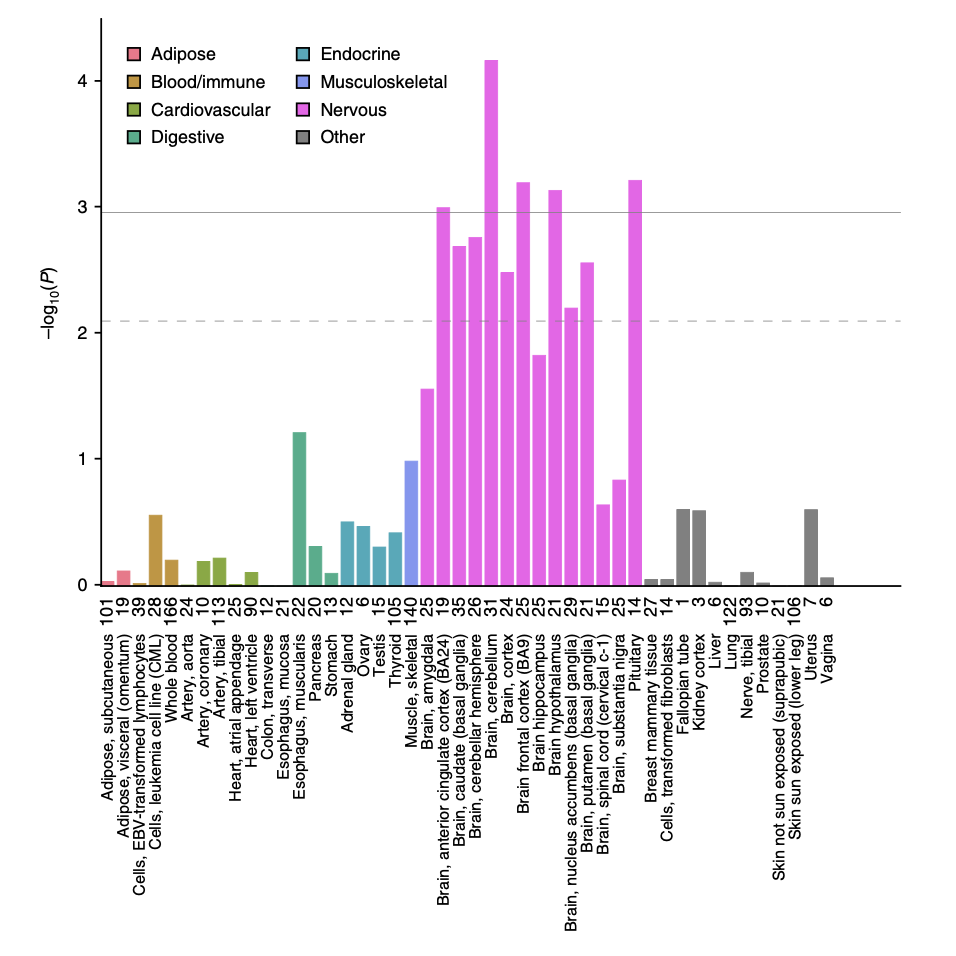
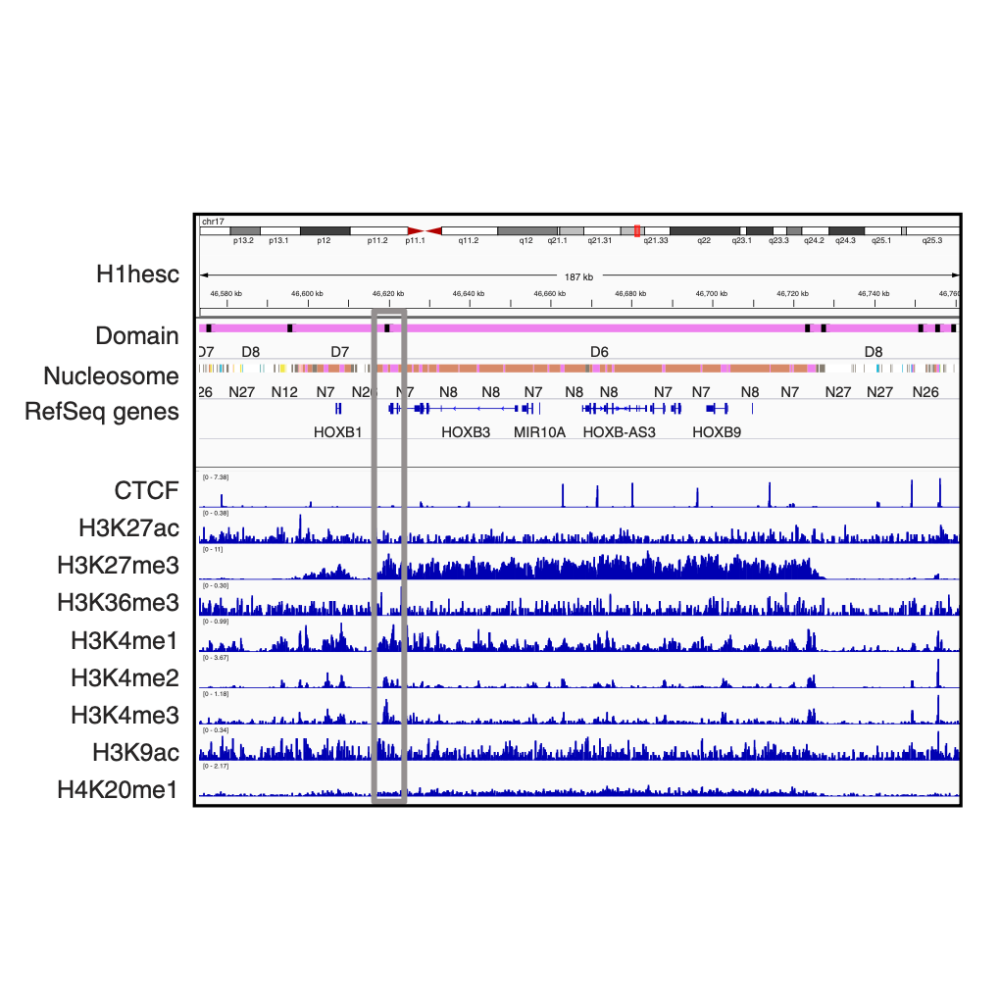
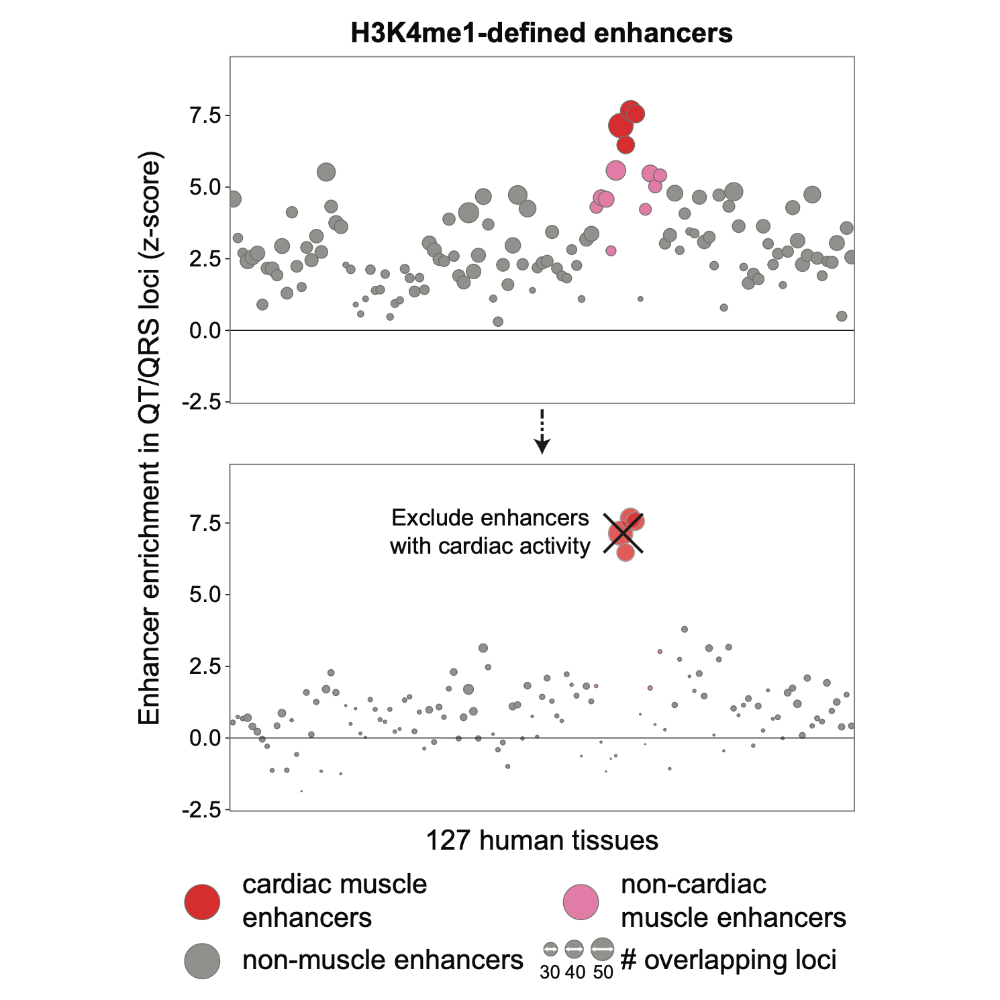
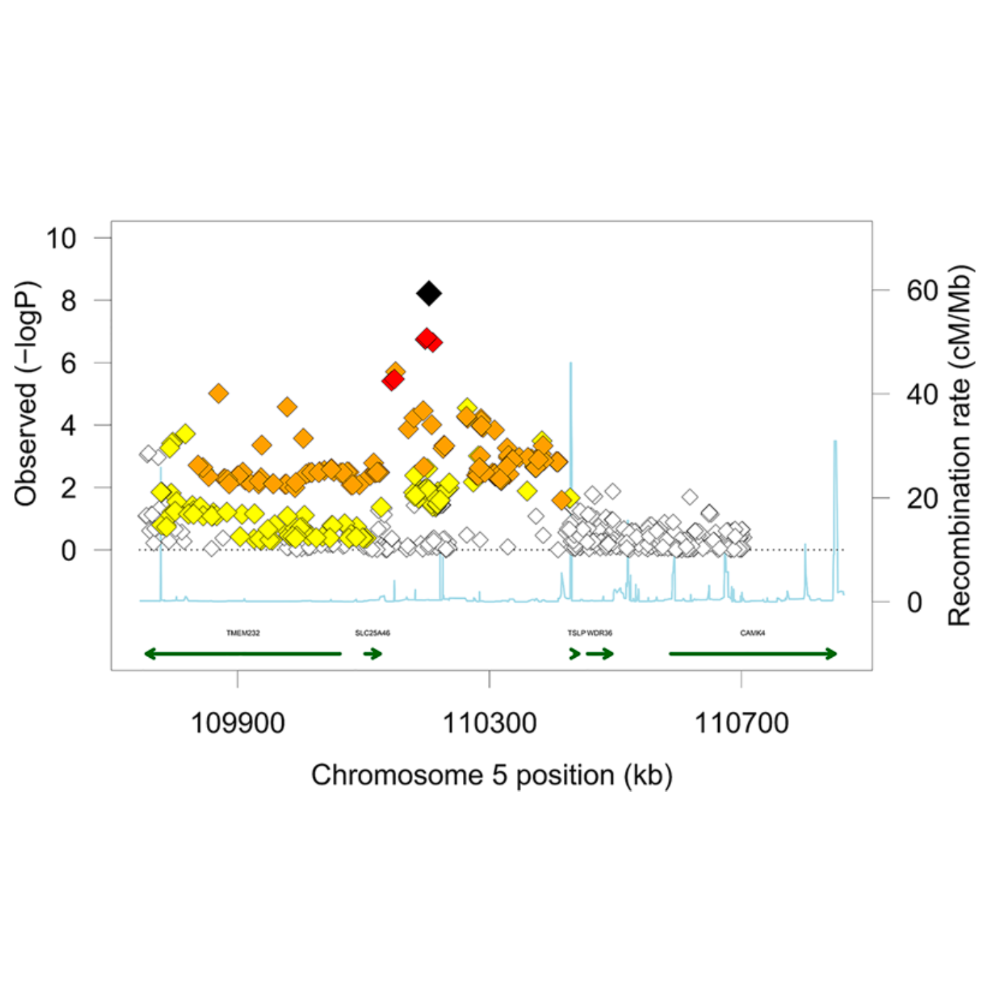
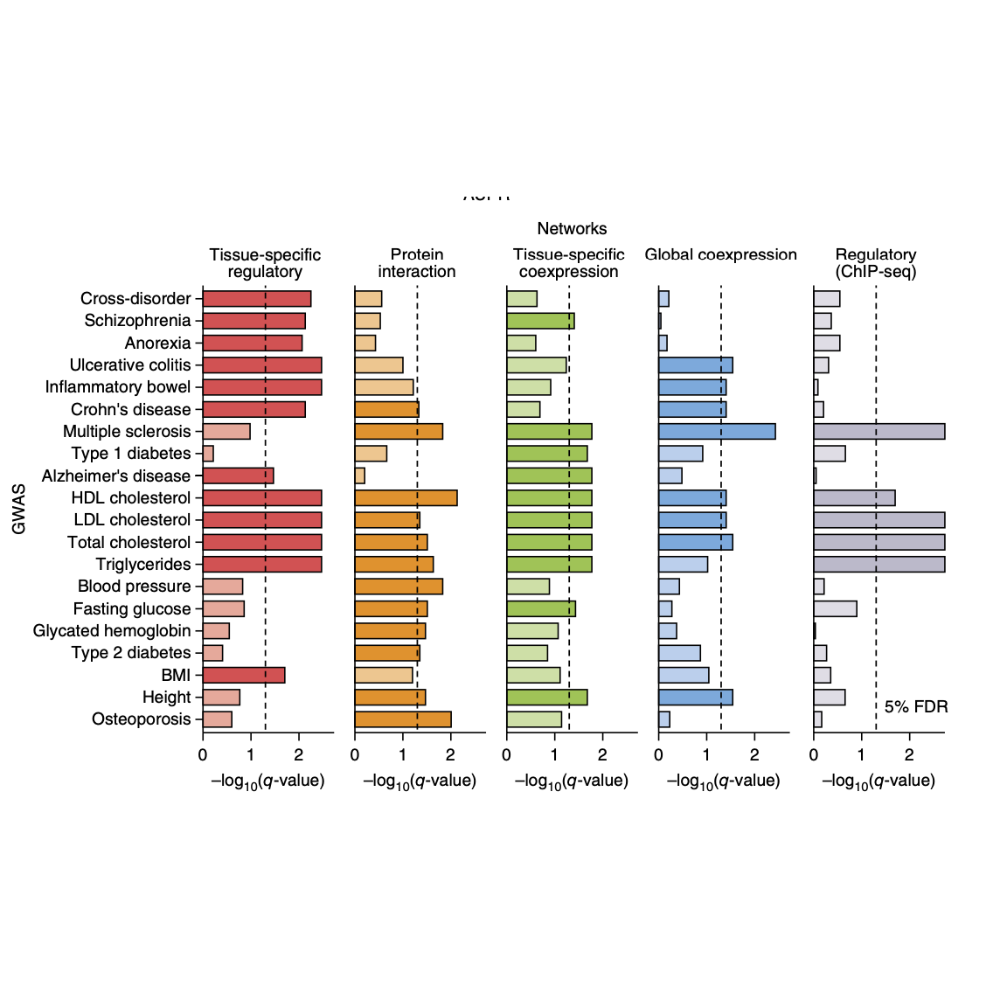
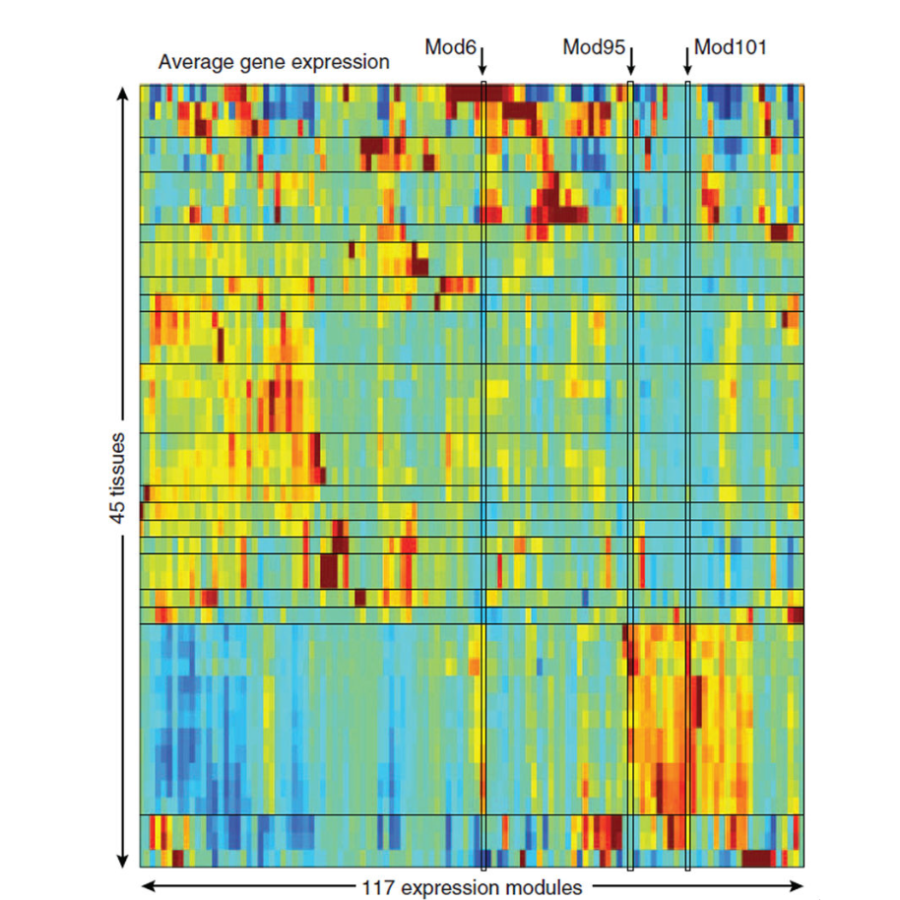
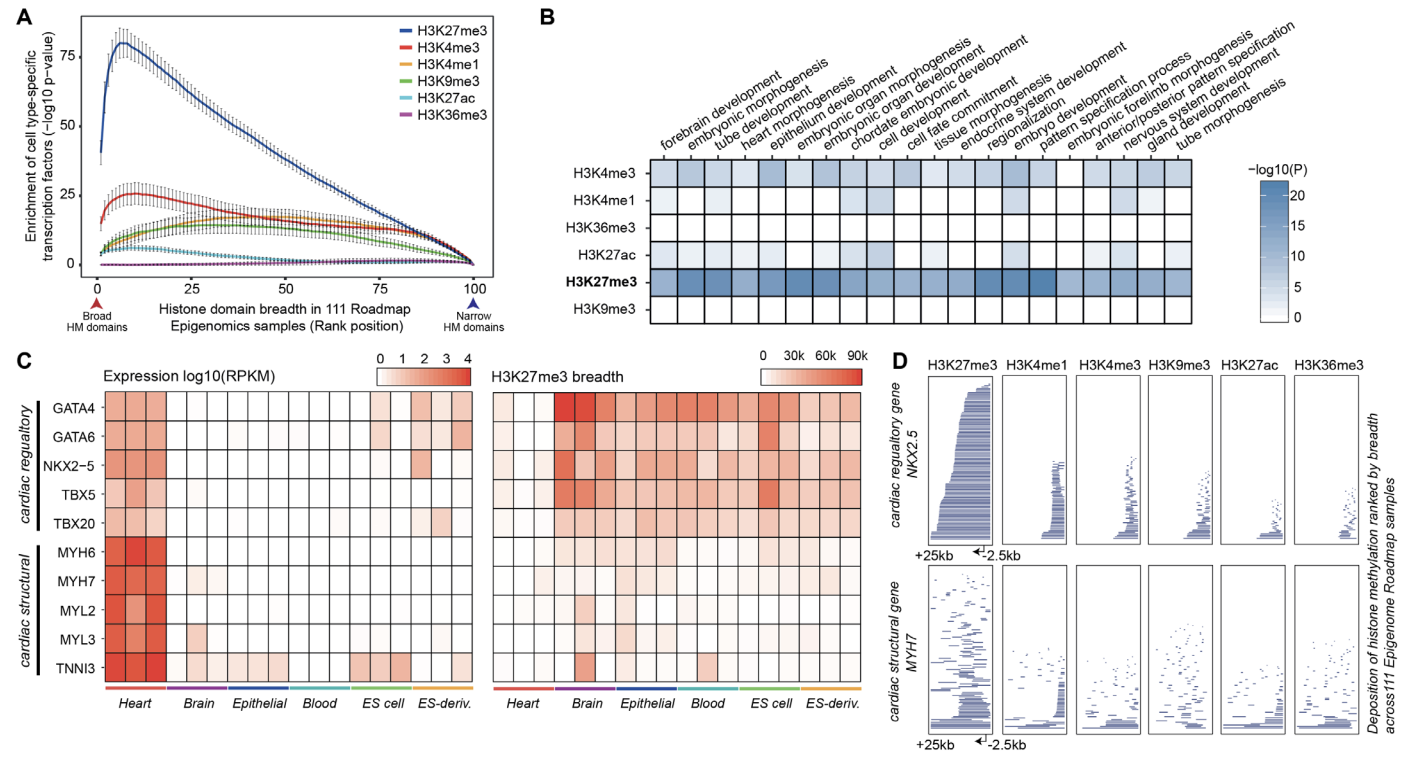
306. Multitissue H3K27ac profiling of GTEx samples links epigenomic variation to disease.
Hou, Xiong, Park, Boix, James, Sun, He, Patel, Zhang, Molinie, Van, Steelman, Nusbaum, Aguet, Ardlie, Kellis
Genetic variants associated with complex traits are primarily noncoding, and their effects on gene-regulatory activity remain largely uncharacterized. To address this, we profile epigenomic variation of histone mark H3K27ac across 387 brain, heart, muscle and lung samples from Genotype-Tissue Expression (GTEx). We annotate 282 k active regulatory elements (AREs) with tissue-specific activity patterns. We identify 2,436 sex-biased AREs and 5,397 genetically influenced AREs associated with 130 k genetic variants (haQTLs) across tissues. We integrate genetic and epigenomic variation to provide mechanistic insights for disease-associated loci from 55 genome-wide association studies (GWAS), by revealing candidate tissues of action, driver SNPs and impacted AREs. Lastly, we build ARE-gene linking scores based on genetics (gLink scores) and demonstrate their unique ability to prioritize SNP-ARE-gene circuits. Overall, our epigenomic datasets, computational integration and mechanistic predictions provide valuable resources and important insights for understanding the molecular basis of human diseases/traits such as schizophrenia.
Journal: Nature Genetics, 2023 Oct DOI: 10.1038/s41588-023-01509-5 PMID: 37770633.0 PMCID: PMC10562256
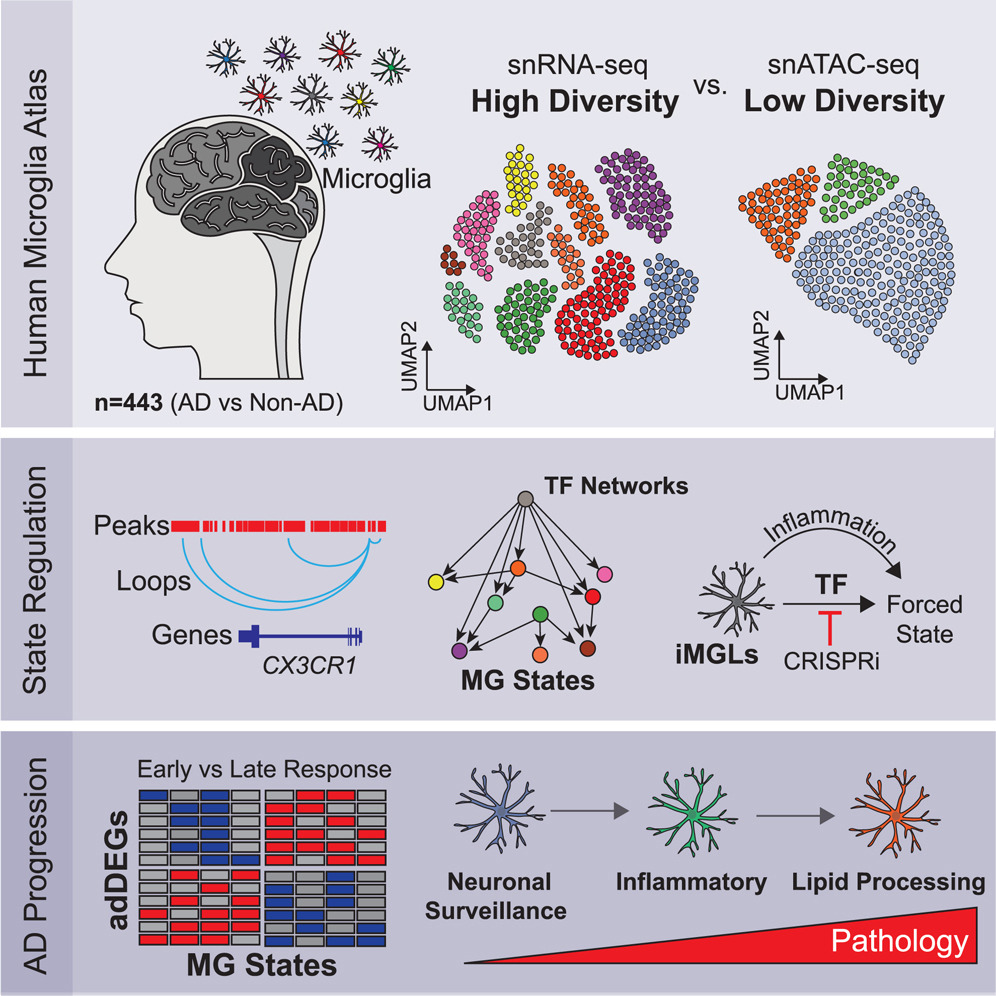
305. Human microglial state dynamics in Alzheimer's disease progression.
Sun, Victor, Park, Xiong, Scannail, Leary, Prosper, Viswanathan, Luna, Boix, James, Tanigawa, Galani, Mathys, Jiang, Ng, Bennett, Tsai, Kellis
Altered microglial states affect neuroinflammation, neurodegeneration, and disease but remain poorly understood. Here, we report 194,000 single-nucleus microglial transcriptomes and epigenomes across 443 human subjects and diverse Alzheimer's disease (AD) pathological phenotypes. We annotate 12 microglial transcriptional states, including AD-dysregulated homeostatic, inflammatory, and lipid-processing states. We identify 1,542 AD-differentially-expressed genes, including both microglia-state-specific and disease-stage-specific alterations. By integrating epigenomic, transcriptomic, and motif information, we infer upstream regulators of microglial cell states, gene-regulatory networks, enhancer-gene links, and transcription-factor-driven microglial state transitions. We demonstrate that ectopic expression of our predicted homeostatic-state activators induces homeostatic features in human iPSC-derived microglia-like cells, while inhibiting activators of inflammation can block inflammatory progression. Lastly, we pinpoint the expression of AD-risk genes in microglial states and differential expression of AD-risk genes and their regulators during AD progression. Overall, we provide insights underlying microglial states, including state-specific and AD-stage-specific microglial alterations at unprecedented resolution.
Journal: Cell, 2023 Sep 28 DOI: 10.1016/j.cell.2023.08.037 PMID: 37774678.0 PMCID: PMC10644954
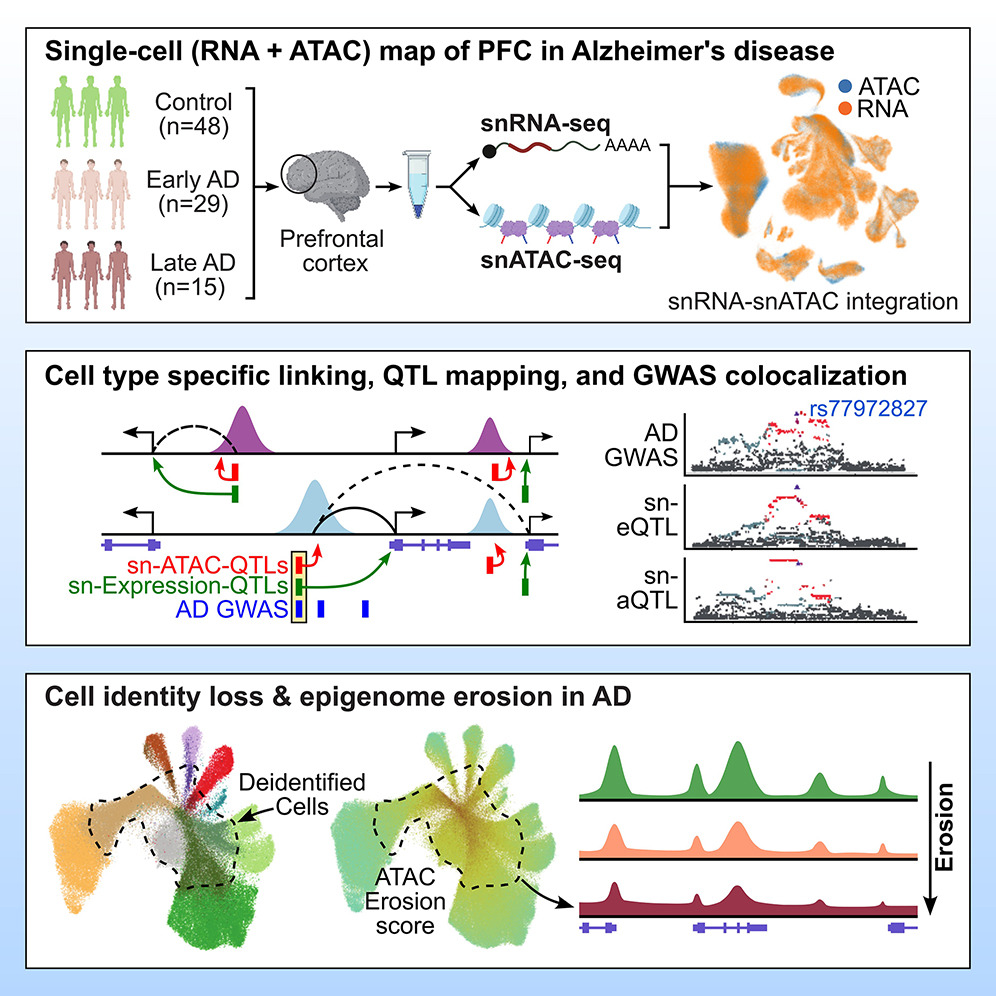
304. Epigenomic dissection of Alzheimer's disease pinpoints causal variants and reveals epigenome erosion.
Xiong, James, Boix, Park, Galani, Victor, Sun, Hou, Ho, Mantero, Scannail, Dileep, Dong, Mathys, Bennett, Tsai, Kellis
Recent work has identified dozens of non-coding loci for Alzheimer's disease (AD) risk, but their mechanisms and AD transcriptional regulatory circuitry are poorly understood. Here, we profile epigenomic and transcriptomic landscapes of 850,000 nuclei from prefrontal cortexes of 92 individuals with and without AD to build a map of the brain regulome, including epigenomic profiles, transcriptional regulators, co-accessibility modules, and peak-to-gene links in a cell-type-specific manner. We develop methods for multimodal integration and detecting regulatory modules using peak-to-gene linking. We show AD risk loci are enriched in microglial enhancers and for specific TFs including SPI1, ELF2, and RUNX1. We detect 9,628 cell-type-specific ATAC-QTL loci, which we integrate alongside peak-to-gene links to prioritize AD variant regulatory circuits. We report differential accessibility of regulatory modules in late AD in glia and in early AD in neurons. Strikingly, late-stage AD brains show global epigenome dysregulation indicative of epigenome erosion and cell identity loss.
Journal: Cell, 2023 Sep 28 DOI: 10.1016/j.cell.2023.08.040 PMID: 37774680.0 PMCID: PMC10782612
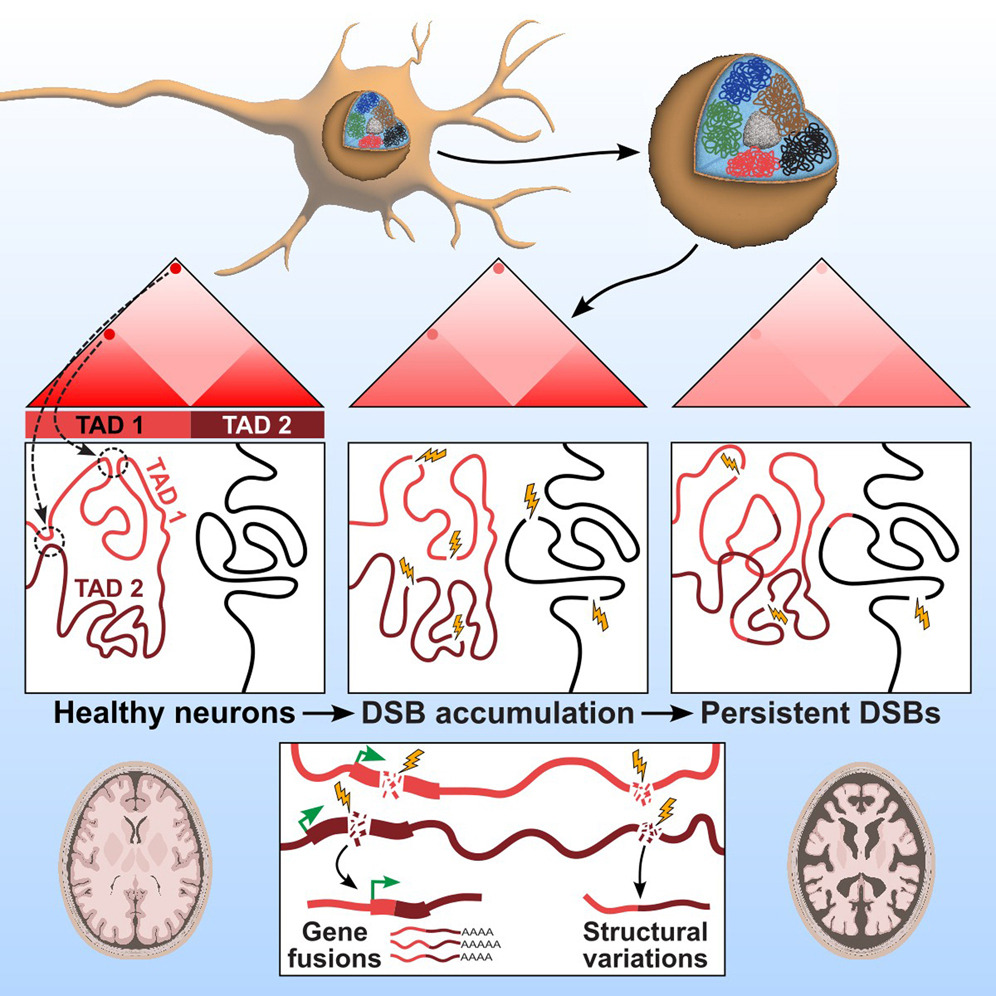
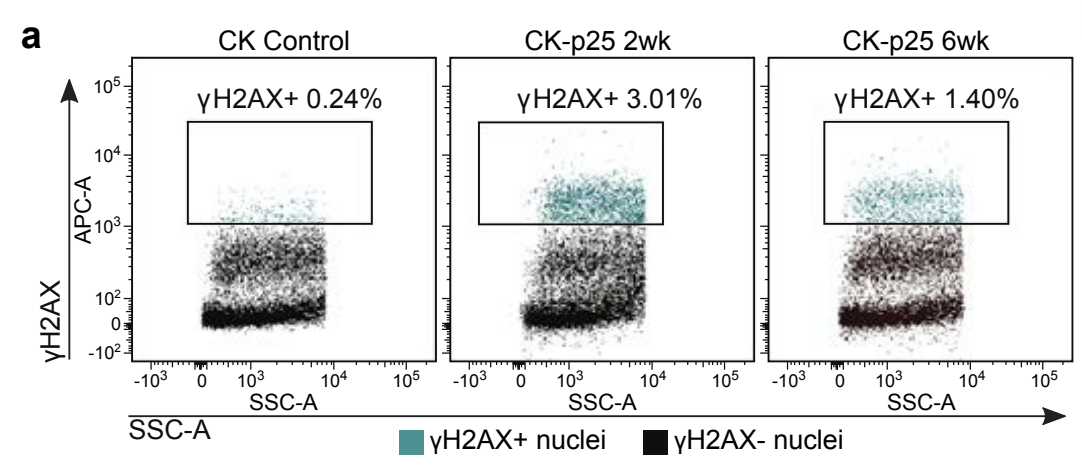
303. Neuronal DNA double-strand breaks lead to genome structural variations and 3D genome disruption in neurodegeneration.
Dileep, Boix, Mathys, Marco, Welch, Meharena, Loon, Jeloka, Peng, Bennett, Kellis, Tsai
Persistent DNA double-strand breaks (DSBs) in neurons are an early pathological hallmark of neurodegenerative diseases including Alzheimer's disease (AD), with the potential to disrupt genome integrity. We used single-nucleus RNA-seq in human postmortem prefrontal cortex samples and found that excitatory neurons in AD were enriched for somatic mosaic gene fusions. Gene fusions were particularly enriched in excitatory neurons with DNA damage repair and senescence gene signatures. In addition, somatic genome structural variations and gene fusions were enriched in neurons burdened with DSBs in the CK-p25 mouse model of neurodegeneration. Neurons enriched for DSBs also had elevated levels of cohesin along with progressive multiscale disruption of the 3D genome organization aligned with transcriptional changes in synaptic, neuronal development, and histone genes. Overall, this study demonstrates the disruption of genome stability and the 3D genome organization by DSBs in neurons as pathological steps in the progression of neurodegenerative diseases.
Journal: Cell, 2023 Sep 28 DOI: 10.1016/j.cell.2023.08.038 PMID: 37774679.0 PMCID: PMC10697236
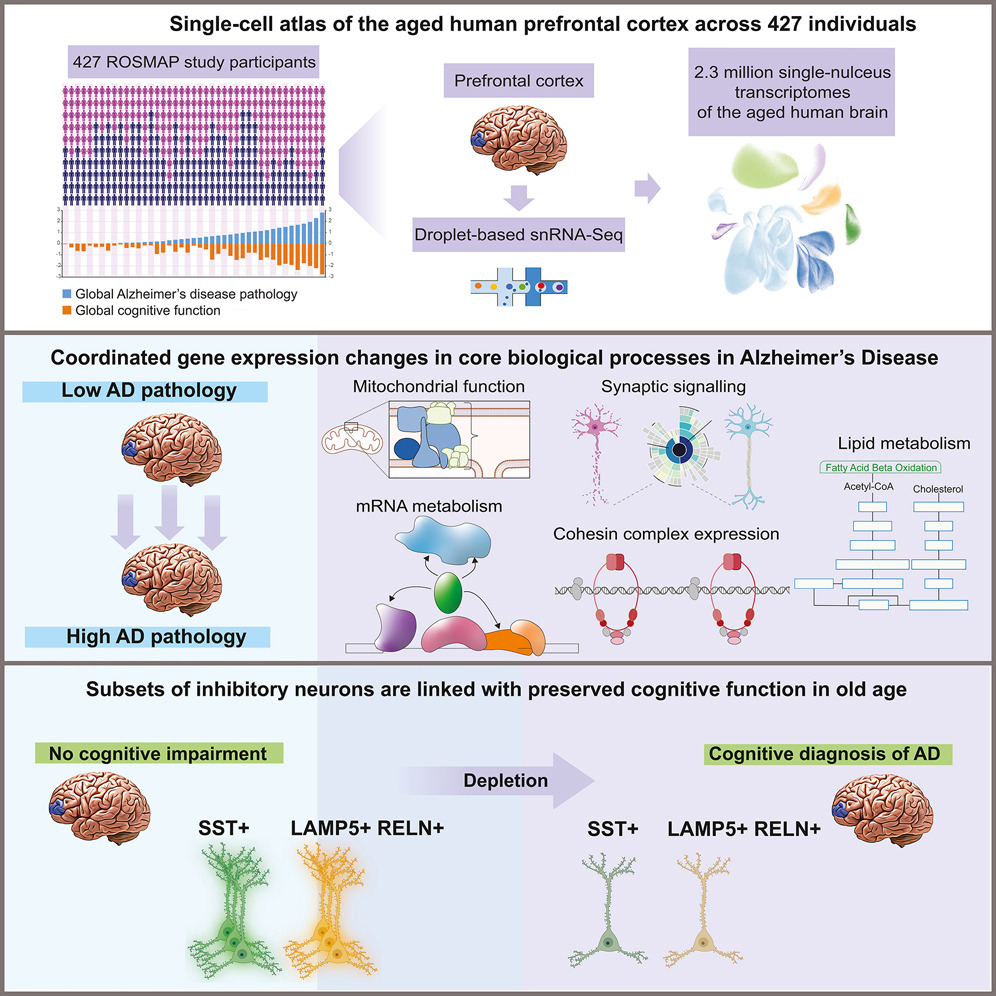
302. Single-cell atlas reveals correlates of high cognitive function, dementia, and resilience to Alzheimer's disease pathology.
Mathys, Peng, Boix, Victor, Leary, Babu, Abdelhady, Jiang, Ng, Ghafari, Kunisky, Mantero, Galani, Lohia, Fortier, Lotfi, Ivey, Brown, Patel, Chakraborty, Beaudway, Imhoff, Keeler, McChesney, Patel, Patel, Thai, Bennett, Kellis, Tsai
Alzheimer's disease (AD) is the most common cause of dementia worldwide, but the molecular and cellular mechanisms underlying cognitive impairment remain poorly understood. To address this, we generated a single-cell transcriptomic atlas of the aged human prefrontal cortex covering 2.3 million cells from postmortem human brain samples of 427 individuals with varying degrees of AD pathology and cognitive impairment. Our analyses identified AD-pathology-associated alterations shared between excitatory neuron subtypes, revealed a coordinated increase of the cohesin complex and DNA damage response factors in excitatory neurons and in oligodendrocytes, and uncovered genes and pathways associated with high cognitive function, dementia, and resilience to AD pathology. Furthermore, we identified selectively vulnerable somatostatin inhibitory neuron subtypes depleted in AD, discovered two distinct groups of inhibitory neurons that were more abundant in individuals with preserved high cognitive function late in life, and uncovered a link between inhibitory neurons and resilience to AD pathology.
Journal: Cell, 2023 Sep 28 DOI: 10.1016/j.cell.2023.08.039 PMID: 37774677.0 PMCID: PMC10601493
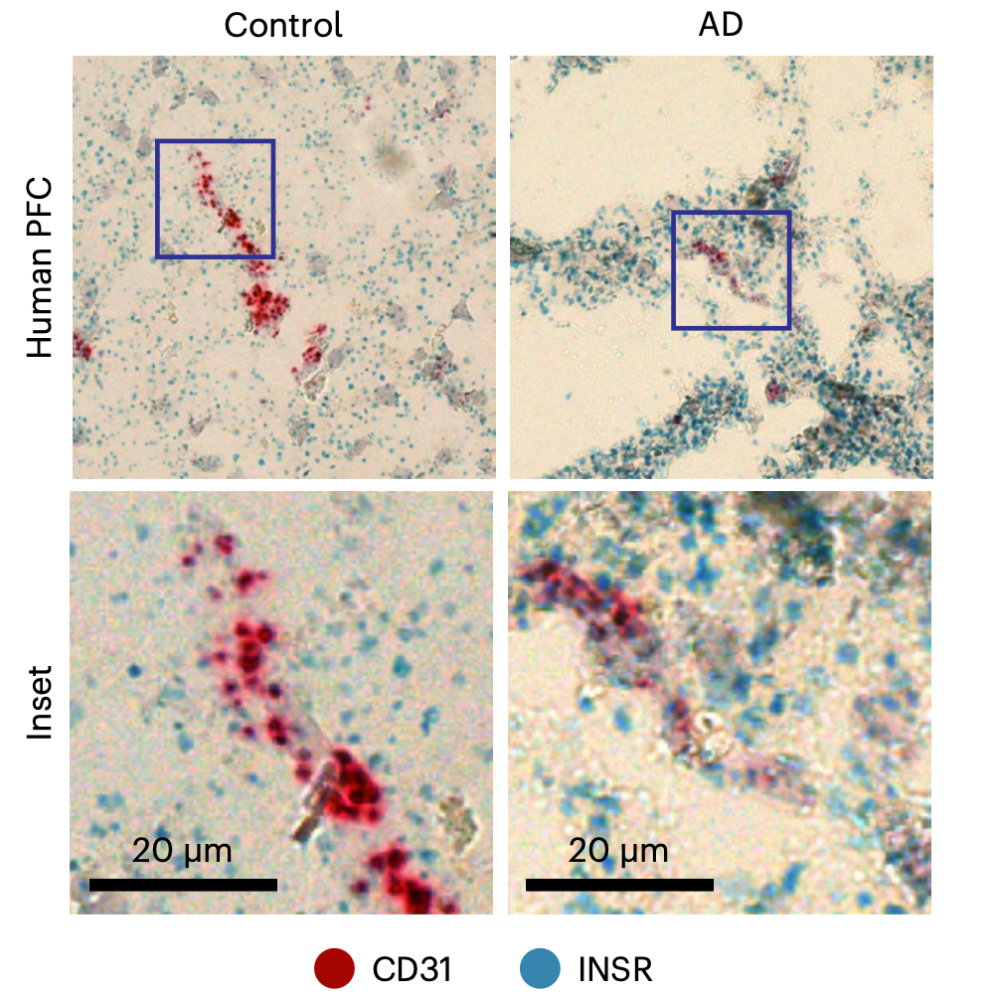
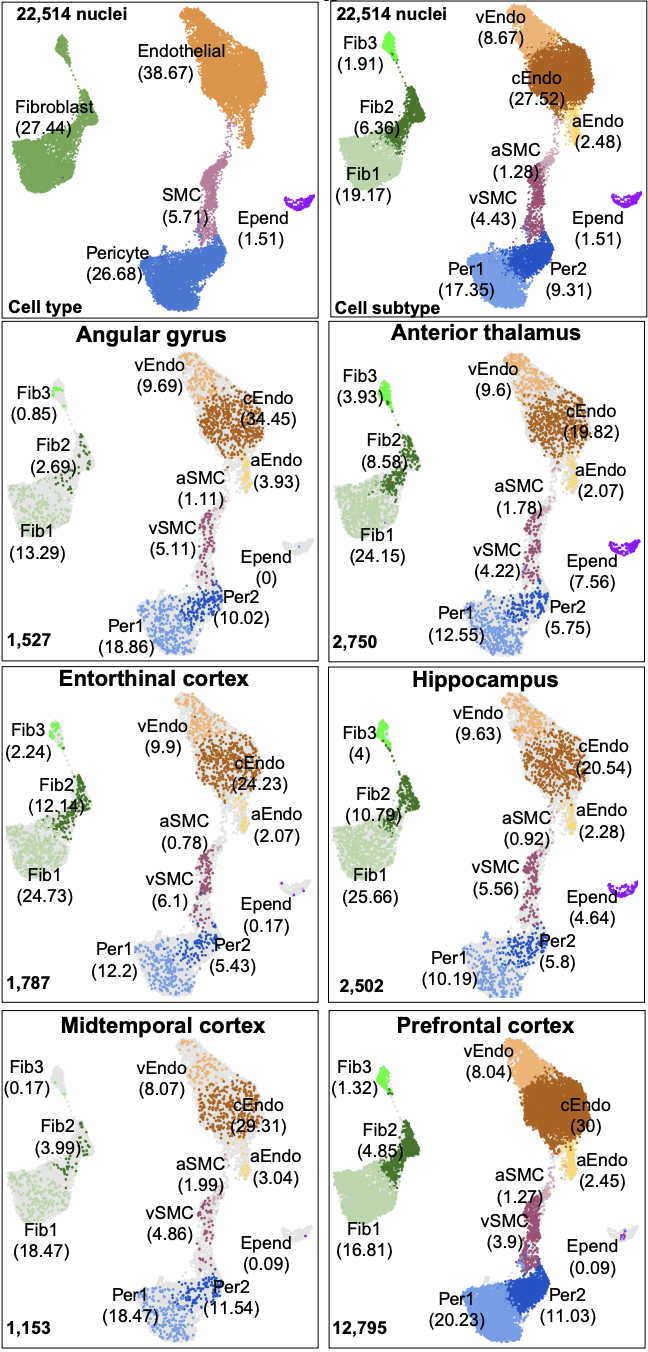
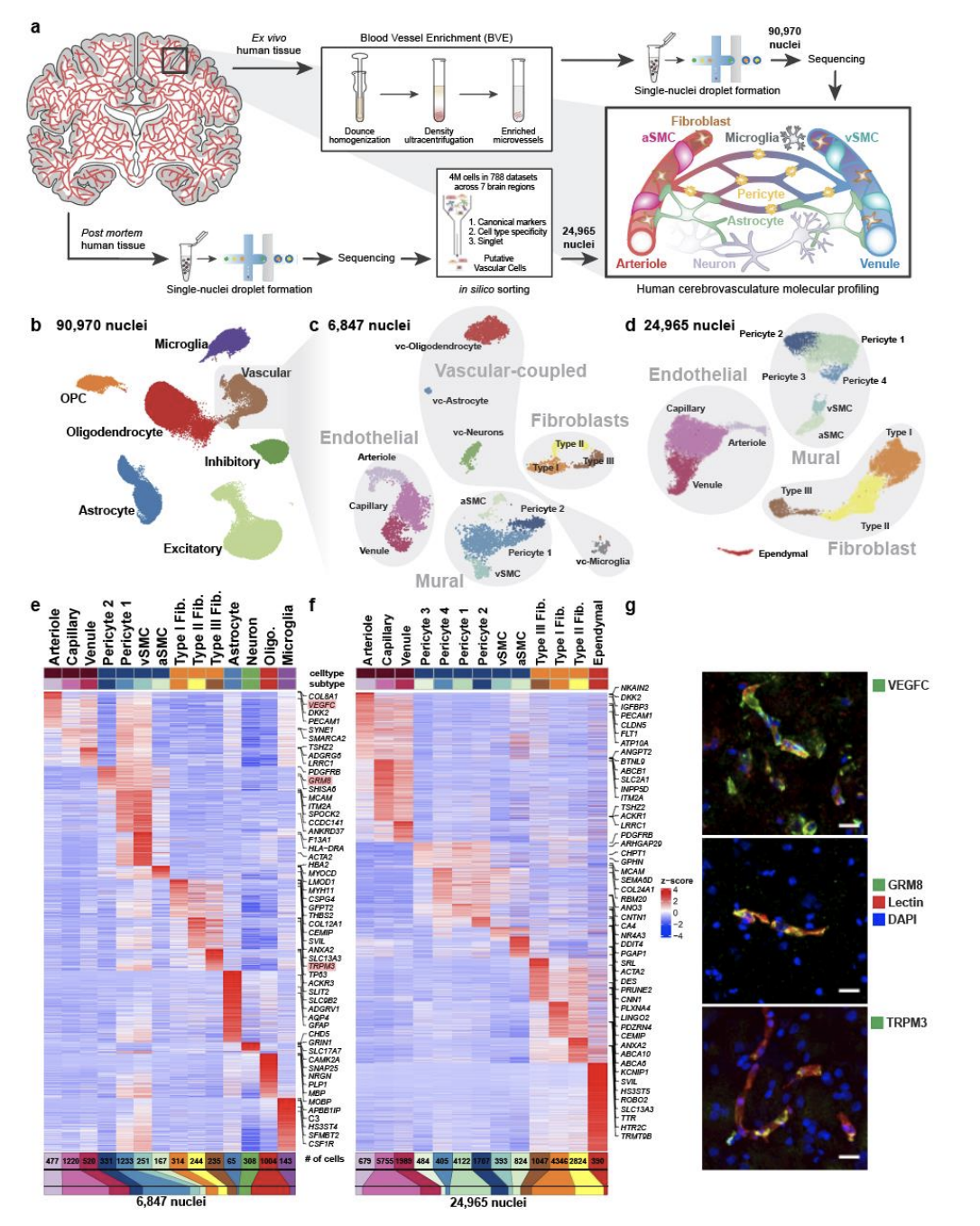
301. Single-nucleus multiregion transcriptomic analysis of brain vasculature in Alzheimer's disease.
Sun, Akay, Murdock, Park, Galiana-Melendez, Bubnys, Galani, Mathys, Jiang, Ng, Bennett, Tsai, Kellis
Cerebrovascular dysregulation is a hallmark of Alzheimer's disease (AD), but the changes that occur in specific cell types have not been fully characterized. Here, we profile single-nucleus transcriptomes in the human cerebrovasculature in six brain regions from 220 individuals with AD and 208 age-matched controls. We annotate 22,514 cerebrovascular cells, including 11 subtypes of endothelial, pericyte, smooth muscle, perivascular fibroblast and ependymal cells. We identify 2,676 differentially expressed genes in AD, including downregulation of PDGFRB in pericytes, and of ABCB1 and ATP10A in endothelial cells, and validate the downregulation of SLC6A1 and upregulation of APOD, INSR and COL4A1 in postmortem AD brain tissues. We detect vasculature, glial and neuronal coexpressed gene modules, suggesting coordinated neurovascular unit dysregulation in AD. Integration with AD genetics reveals 125 AD differentially expressed genes directly linked to AD-associated genetic variants. Lastly, we show that APOE4 genotype-associated differences are significantly enriched among AD-associated genes in capillary and venule endothelial cells, as well as subsets of pericytes and fibroblasts.
Journal: Nature Neuroscience, 2023 Jun DOI: 10.1038/s41593-023-01334-3 PMID: 37264161.0 PMCID: PMC10464935
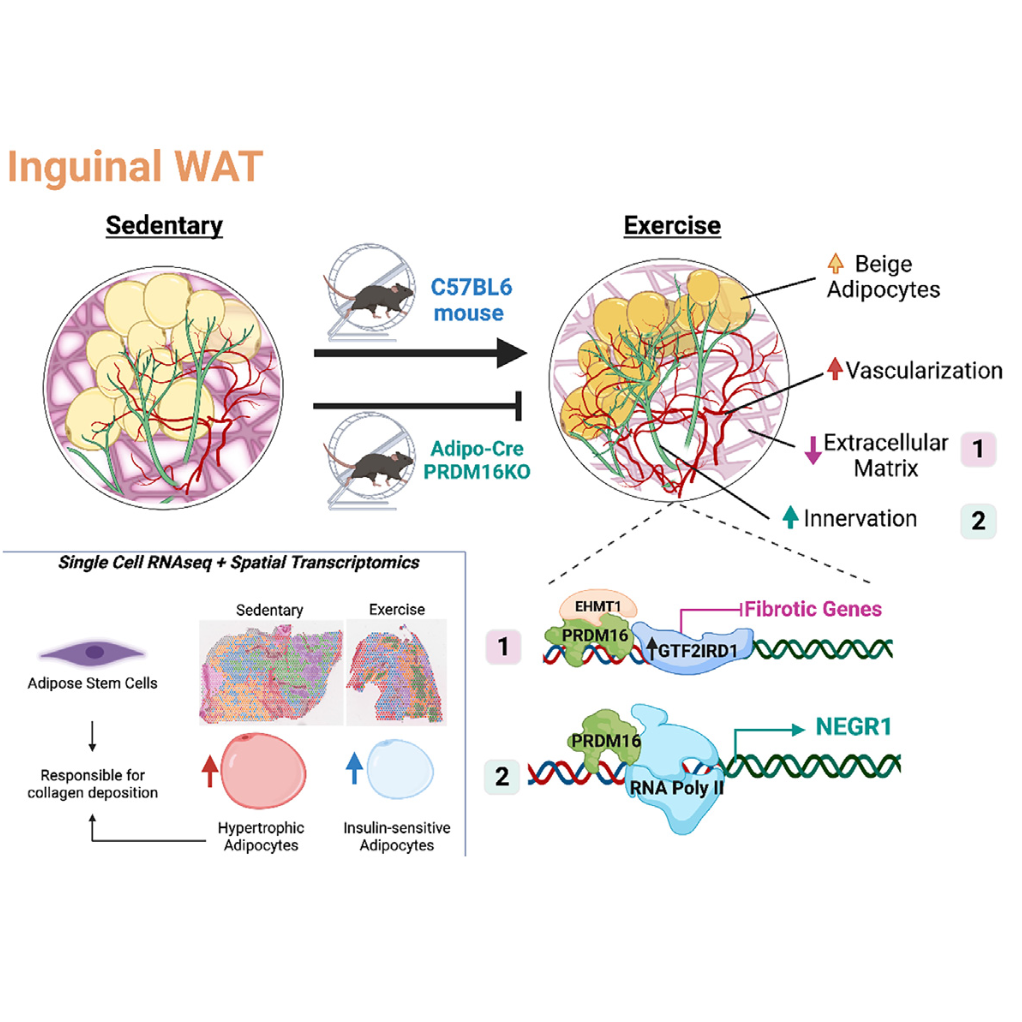
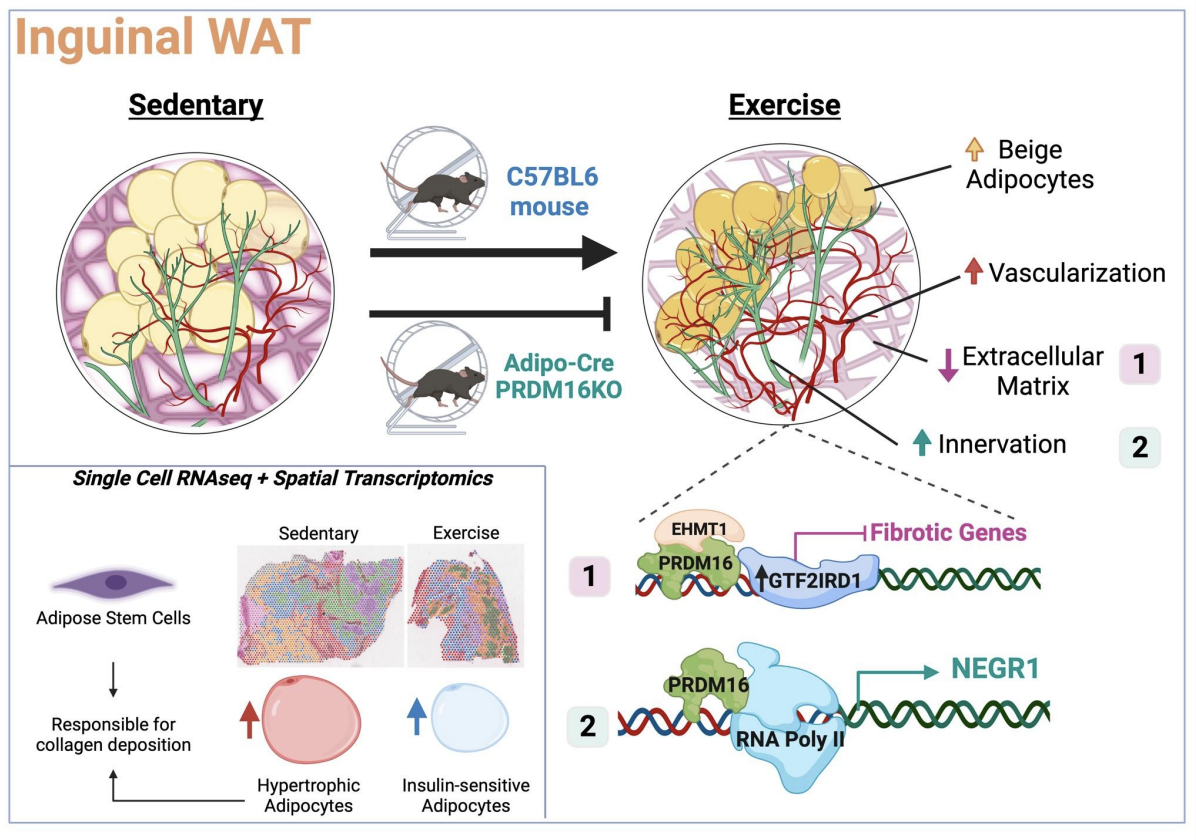
300. Exercise training remodels inguinal white adipose tissue through adaptations in innervation, vascularization, and the extracellular matrix.
Nigro, Vamvini, Yang, Caputo, Ho, Carbone, Papadopoulos, Conlin, He, Hirshman, White, Robidoux, Hickner, Nielsen, Pedersen, Kellis, Middelbeek, Goodyear
Inguinal white adipose tissue (iWAT) is essential for the beneficial effects of exercise training on metabolic health. The underlying mechanisms for these effects are not fully understood, and here, we test the hypothesis that exercise training results in a more favorable iWAT structural phenotype. Using biochemical, imaging, and multi-omics analyses, we find that 11 days of wheel running in male mice causes profound iWAT remodeling including decreased extracellular matrix (ECM) deposition and increased vascularization and innervation. We identify adipose stem cells as one of the main contributors to training-induced ECM remodeling, show that the PRDM16 transcriptional complex is necessary for iWAT remodeling and beiging, and discover neuronal growth regulator 1 (NEGR1) as a link between PRDM16 and neuritogenesis. Moreover, we find that training causes a shift from hypertrophic to insulin-sensitive adipocyte subpopulations. Exercise training leads to remarkable adaptations to iWAT structure and cell-type composition that can confer beneficial changes in tissue metabolism.
Journal: Cell Reports, 2023 Apr 25 DOI: 10.1016/j.celrep.2023.112392 PMID: 37058410.0 PMCID: PMC10374102
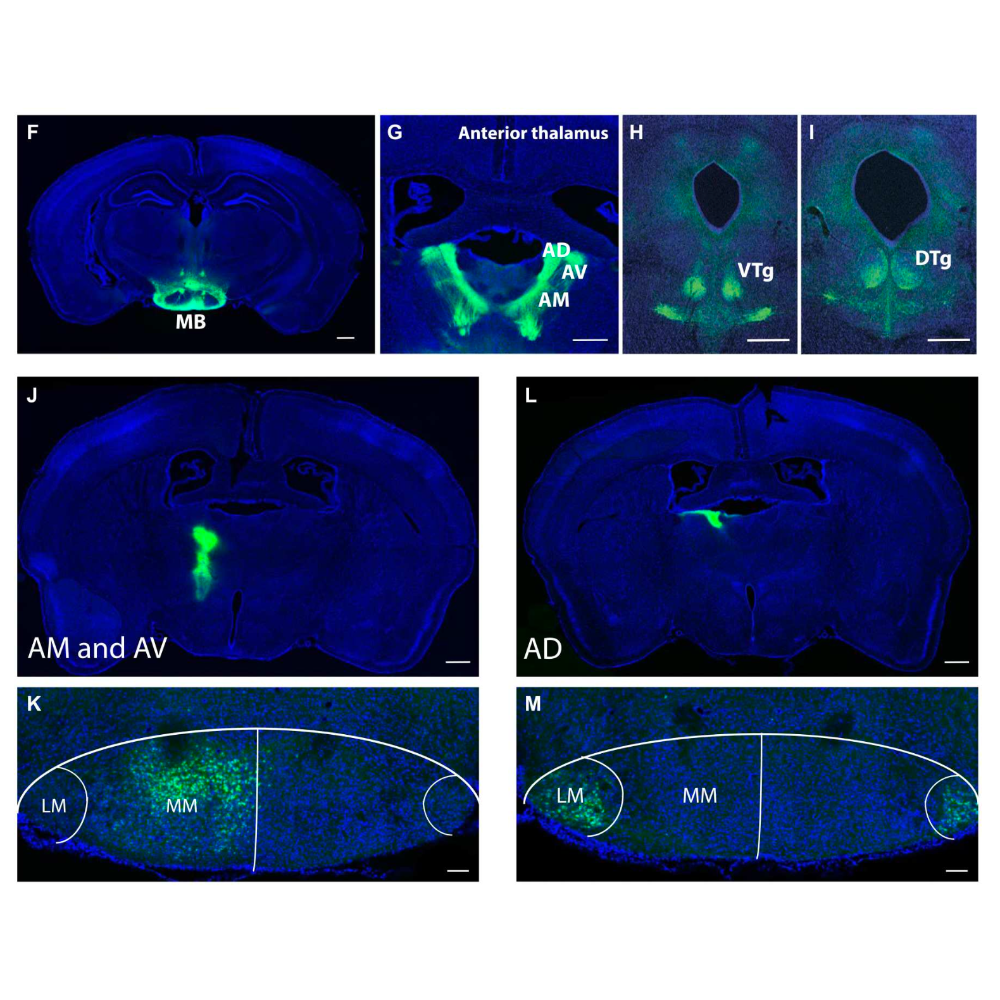
299. Lateral mammillary body neurons in mouse brain are disproportionately vulnerable in Alzheimer's disease.
Huang, Peng, Murdock, Liu, Mathys, Davila-Velderrain, Jiang, Chen, Ng, Kim, Abdurrob, Gao, Bennett, Kellis, Tsai
The neural circuits governing the induction and progression of neurodegeneration and memory impairment in Alzheimer's disease (AD) are incompletely understood. The mammillary body (MB), a subcortical node of the medial limbic circuit, is one of the first brain regions to exhibit amyloid deposition in the 5xFAD mouse model of AD. Amyloid burden in the MB correlates with pathological diagnosis of AD in human postmortem brain tissue. Whether and how MB neuronal circuitry contributes to neurodegeneration and memory deficits in AD are unknown. Using 5xFAD mice and postmortem MB samples from individuals with varying degrees of AD pathology, we identified two neuronal cell types in the MB harboring distinct electrophysiological properties and long-range projections: lateral neurons and medial neurons. lateral MB neurons harbored aberrant hyperactivity and exhibited early neurodegeneration in 5xFAD mice compared with lateral MB neurons in wild-type littermates. Inducing hyperactivity in lateral MB neurons in wild-type mice impaired performance on memory tasks, whereas attenuating aberrant hyperactivity in lateral MB neurons ameliorated memory deficits in 5xFAD mice. Our findings suggest that neurodegeneration may be a result of genetically distinct, projection-specific cellular dysfunction and that dysregulated lateral MB neurons may be causally linked to memory deficits in AD.
Journal: Science Translational Medicine, 2023 Apr 19 DOI: 10.1126/scitranslmed.abq1019 PMID: 37075128.0 PMCID: PMC10511020
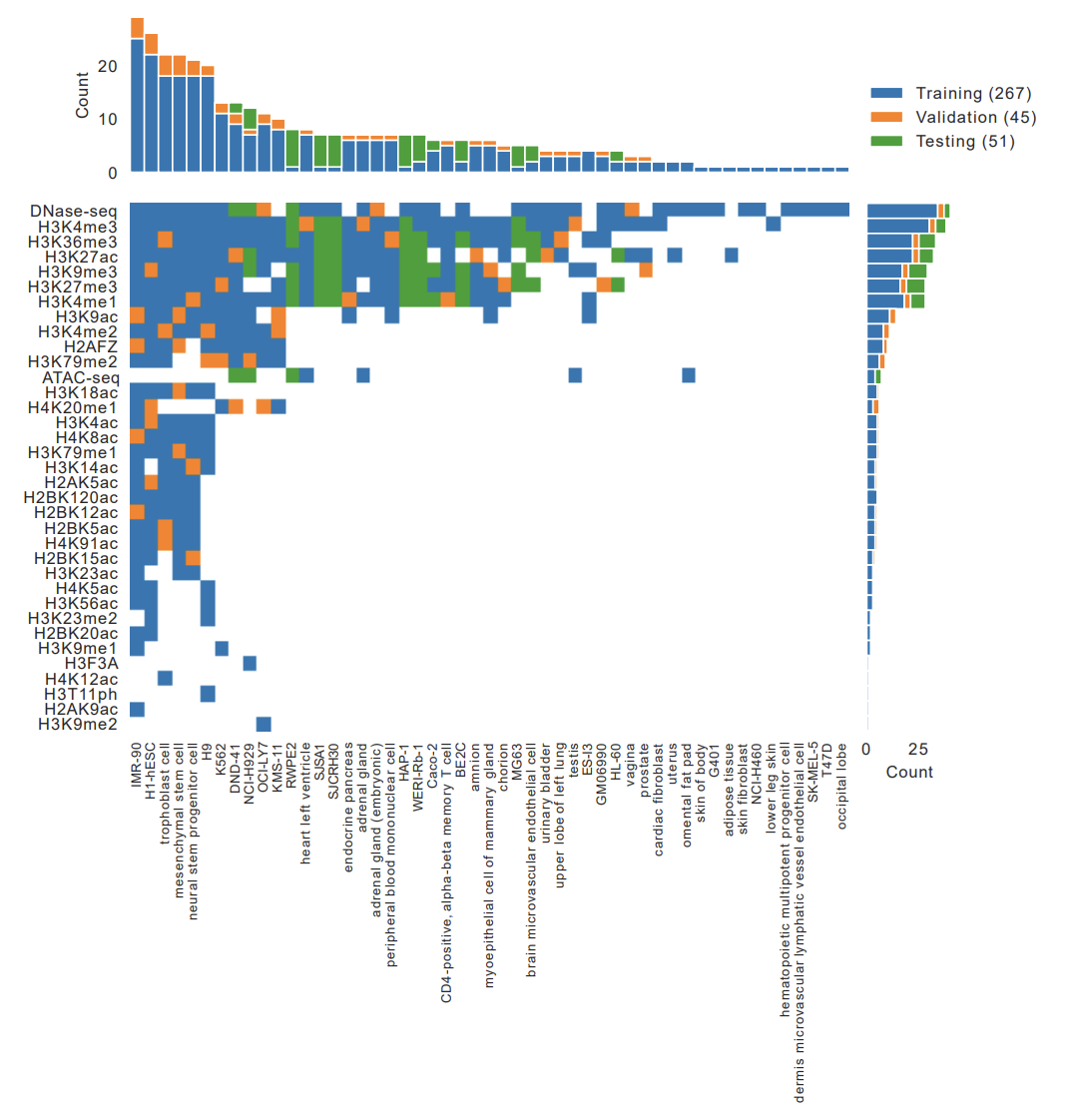
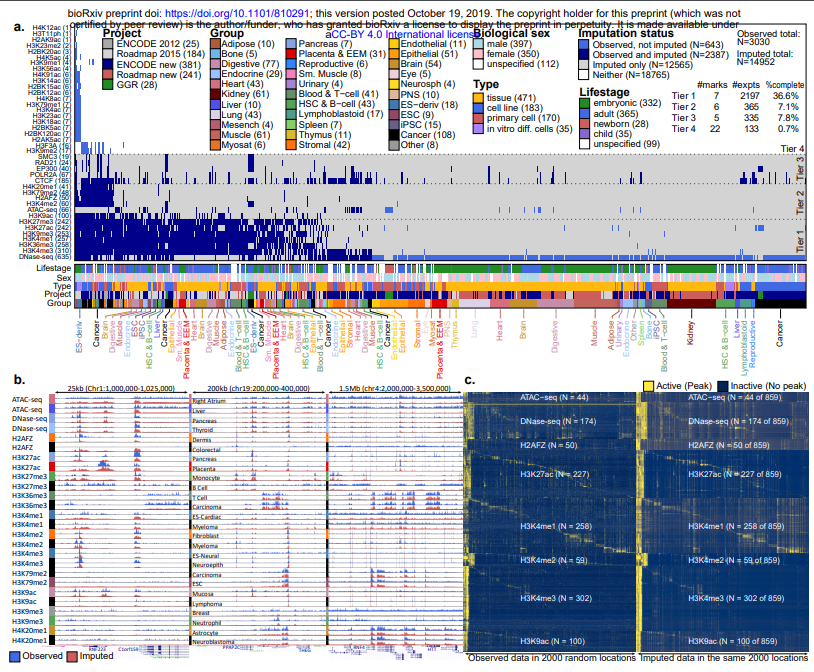
298. The ENCODE Imputation Challenge: a critical assessment of methods for cross-cell type imputation of epigenomic profiles.
Schreiber, Boix, Wook, Li, Guan, Chang, Chang, Hawkins-Hooker, Scholkopf, Schweikert, Carulla, Canakoglu, Guzzo, Nanni, Masseroli, Carman, Pinoli, Hong, Yip, Spence, Batra, Song, Mahony, Zhang, Tan, Shen, Sun, Shi, Adrian, Sandstrom, Farrell, Halow, Lee, Jiang, Yang, Epstein, Strattan, Bernstein, Snyder, Kellis, Stafford, Kundaje
A promising alternative to comprehensively performing genomics experiments is to, instead, perform a subset of experiments and use computational methods to impute the remainder. However, identifying the best imputation methods and what measures meaningfully evaluate performance are open questions. We address these questions by comprehensively analyzing 23 methods from the ENCODE Imputation Challenge. We find that imputation evaluations are challenging and confounded by distributional shifts from differences in data collection and processing over time, the amount of available data, and redundancy among performance measures. Our analyses suggest simple steps for overcoming these issues and promising directions for more robust research.
Journal: Genome Biology, 2023 Apr 18 DOI: 10.1186/s13059-023-02915-y PMID: 37072822.0 PMCID: PMC10111747
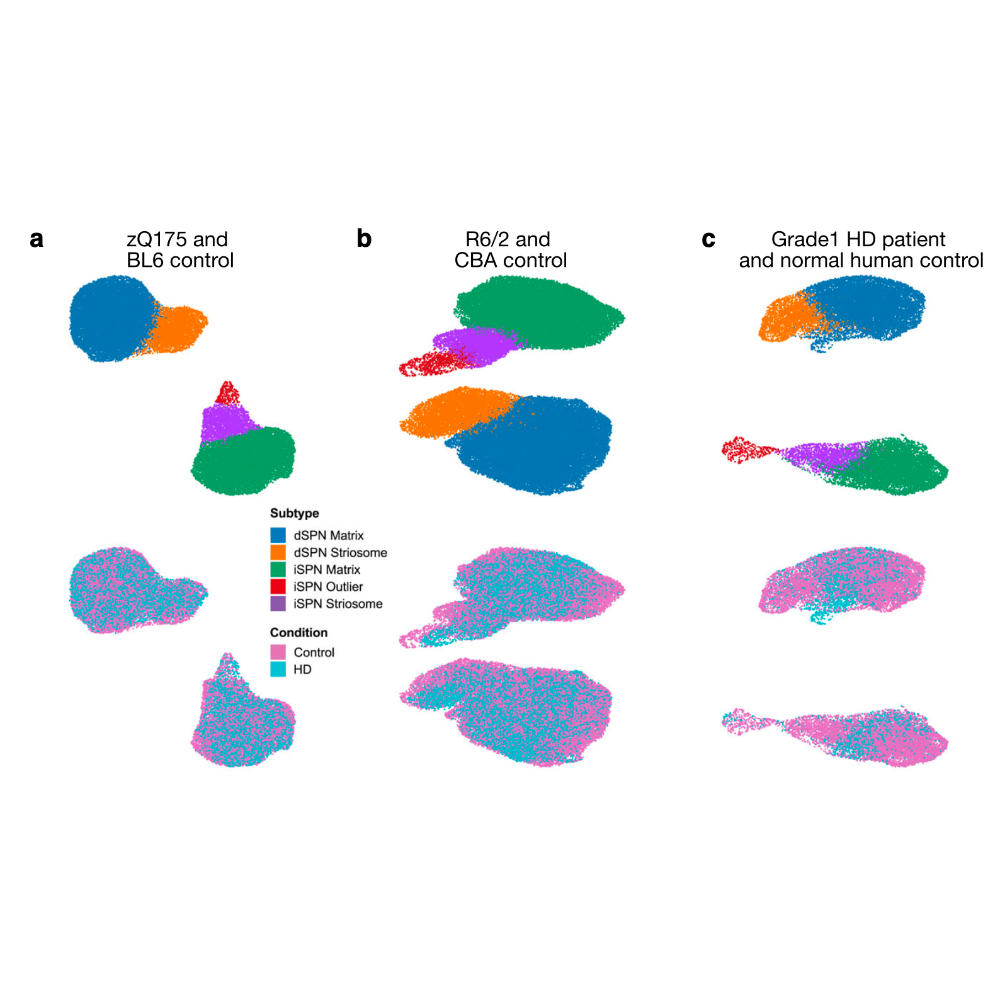
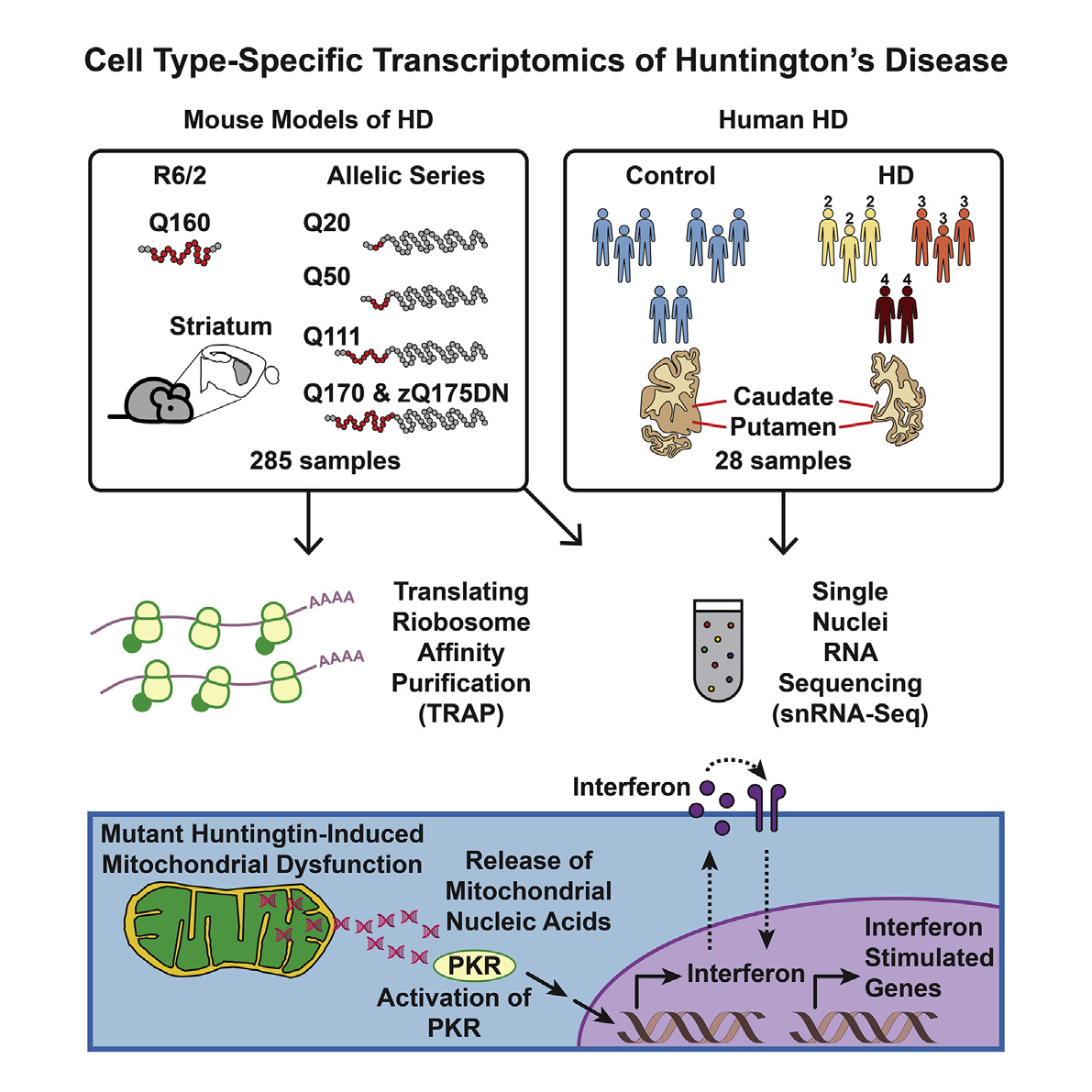
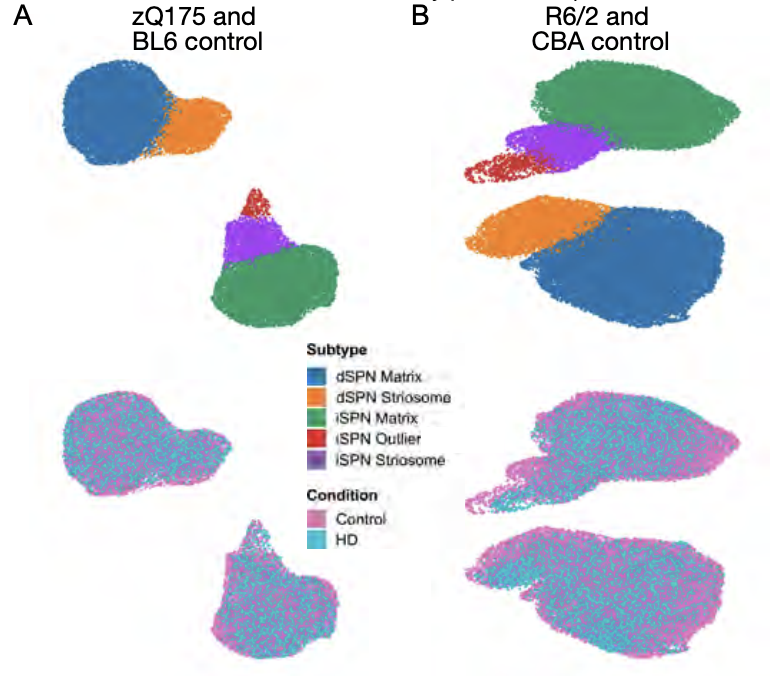
297. Transcriptional vulnerabilities of striatal neurons in human and rodent models of Huntington's disease.
Matsushima, Pineda, Crittenden, Lee, Galani, Mantero, Tombaugh, Kellis, Heiman, Graybiel
Striatal projection neurons (SPNs), which progressively degenerate in human patients with Huntington's disease (HD), are classified along two axes: the canonical direct-indirect pathway division and the striosome-matrix compartmentation. It is well established that the indirect-pathway SPNs are susceptible to neurodegeneration and transcriptomic disturbances, but less is known about how the striosome-matrix axis is compromised in HD in relation to the canonical axis. Here we show, using single-nucleus RNA-sequencing data from male Grade 1 HD patient post-mortem brain samples and male zQ175 and R6/2 mouse models, that the two axes are multiplexed and differentially compromised in HD. In human HD, striosomal indirect-pathway SPNs are the most depleted SPN population. In mouse HD models, the transcriptomic distinctiveness of striosome-matrix SPNs is diminished more than that of direct-indirect pathway SPNs. Furthermore, the loss of striosome-matrix distinction is more prominent within indirect-pathway SPNs. These results open the possibility that the canonical direct-indirect pathway and striosome-matrix compartments are differentially compromised in late and early stages of disease progression, respectively, differentially contributing to the symptoms, thus calling for distinct therapeutic strategies.
Journal: Nature Communications, 2023 Jan 17 DOI: 10.1038/s41467-022-35752-x PMID: 36650127.0 PMCID: PMC9845362
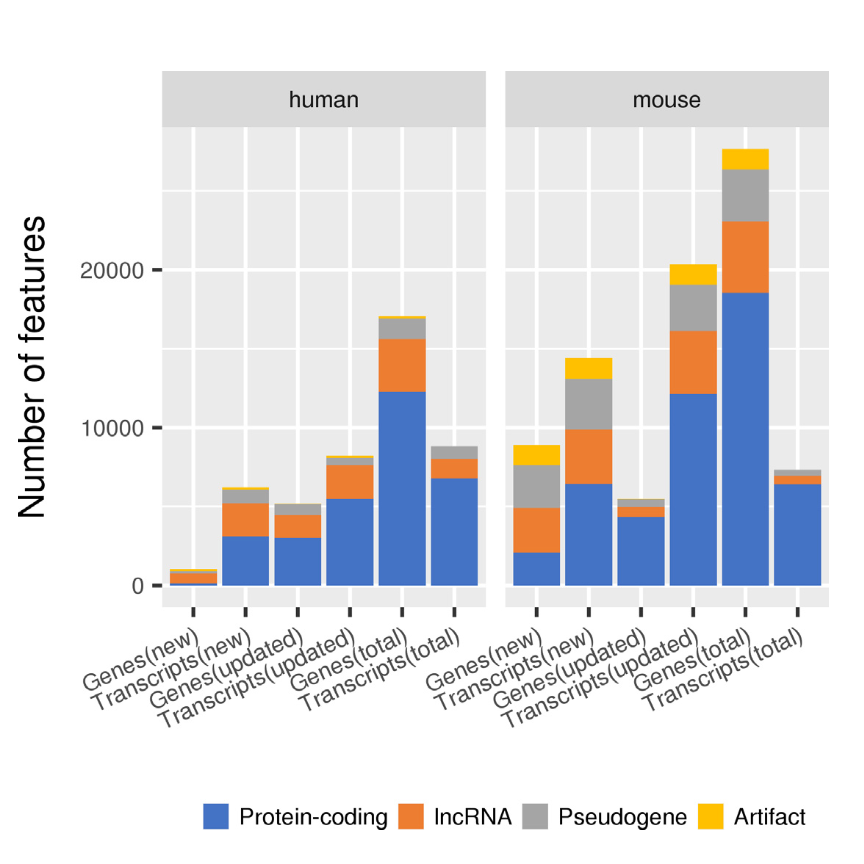
296. GENCODE: reference annotation for the human and mouse genomes in 2023.
Frankish, Carbonell-Sala, Diekhans, Jungreis, Loveland, Mudge, Sisu, Wright, Arnan, Barnes, Banerjee, Bennett, Berry, Bignell, Boix, Calvet, Cerdan-Velez, Cunningham, Davidson, Donaldson, Dursun, Fatima, Giorgetti, Giron, Gonzalez, Hardy, Harrison, Hourlier, Hollis, Hunt, James, Jiang, Johnson, Kay, Lagarde, Martin, Gomez, Nair, Ni, Pozo, Ramalingam, Ruffier, Schmitt, Schreiber, Steed, Suner, Sumathipala, Sycheva, Uszczynska-Ratajczak, Wass, Yang, Yates, Zafrulla, Choudhary, Gerstein, Guigo, Hubbard, Kellis, Kundaje, Paten, Tress, Flicek
GENCODE produces high quality gene and transcript annotation for the human and mouse genomes. All GENCODE annotation is supported by experimental data and serves as a reference for genome biology and clinical genomics. The GENCODE consortium generates targeted experimental data, develops bioinformatic tools and carries out analyses that, along with externally produced data and methods, support the identification and annotation of transcript structures and the determination of their function. Here, we present an update on the annotation of human and mouse genes, including developments in the tools, data, analyses and major collaborations which underpin this progress. For example, we report the creation of a set of non-canonical ORFs identified in GENCODE transcripts, the LRGASP collaboration to assess the use of long transcriptomic data to build transcript models, the progress in collaborations with RefSeq and UniProt to increase convergence in the annotation of human and mouse protein-coding genes, the propagation of GENCODE across the human pan-genome and the development of new tools to support annotation of regulatory features by GENCODE. Our annotation is accessible via Ensembl, the UCSC Genome Browser and https://www.gencodegenes.org.
Journal: Nucleic Acids Research, 2023 Jan 6 DOI: 10.1093/nar/gkac1071 PMID: 36420896.0 PMCID: PMC9825462
295. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes.
Blanchard, Akay, Davila-Velderrain, von, Mathys, Davidson, Effenberger, Chen, Maner-Smith, Hajjar, Ortlund, Bula, Agbas, Ng, Jiang, Kahn, Blanco-Duque, Lavoie, Liu, Reyes, Lin, Ko, R'Bibo, Ralvenius, Bennett, Cam, Kellis, Tsai
APOE4 is the strongest genetic risk factor for Alzheimer's disease(1-3). However, the effects of APOE4 on the human brain are not fully understood, limiting opportunities to develop targeted therapeutics for individuals carrying APOE4 and other risk factors for Alzheimer's disease(4-8). Here, to gain more comprehensive insights into the impact of APOE4 on the human brain, we performed single-cell transcriptomics profiling of post-mortem human brains from APOE4 carriers compared with non-carriers. This revealed that APOE4 is associated with widespread gene expression changes across all cell types of the human brain. Consistent with the biological function of APOE(2-6), APOE4 significantly altered signalling pathways associated with cholesterol homeostasis and transport. Confirming these findings with histological and lipidomic analysis of the post-mortem human brain, induced pluripotent stem-cell-derived cells and targeted-replacement mice, we show that cholesterol is aberrantly deposited in oligodendrocytes-myelinating cells that are responsible for insulating and promoting the electrical activity of neurons. We show that altered cholesterol localization in the APOE4 brain coincides with reduced myelination. Pharmacologically facilitating cholesterol transport increases axonal myelination and improves learning and memory in APOE4 mice. We provide a single-cell atlas describing the transcriptional effects of APOE4 on the aging human brain and establish a functional link between APOE4, cholesterol, myelination and memory, offering therapeutic opportunities for Alzheimer's disease.
Journal: Nature, 2022 Nov DOI: 10.1038/s41586-022-05439-w PMID: 36385529.0 PMCID: PMC9870060
294. A synthetic transcription platform for programmable gene expression in mammalian cells.
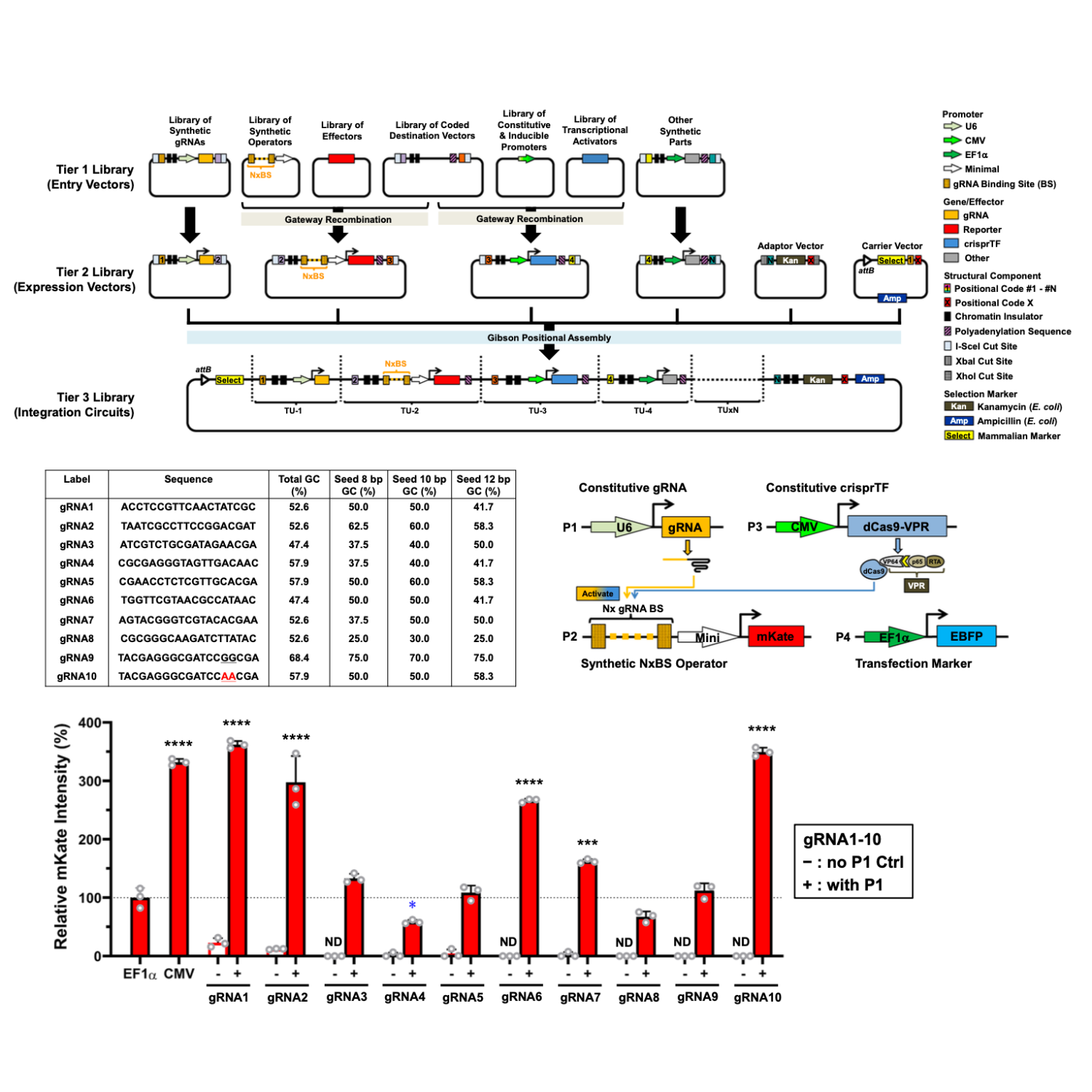
Chen, Gaidukov, Lai, Wu, Cao, Gutbrod, Choi, Utomo, Chen, Wroblewska, Kellis, Zhang, Weiss, Lu
Precise, scalable, and sustainable control of genetic and cellular activities in mammalian cells is key to developing precision therapeutics and smart biomanufacturing. Here we create a highly tunable, modular, versatile CRISPR-based synthetic transcription system for the programmable control of gene expression and cellular phenotypes in mammalian cells. Genetic circuits consisting of well-characterized libraries of guide RNAs, binding motifs of synthetic operators, transcriptional activators, and additional genetic regulatory elements express mammalian genes in a highly predictable and tunable manner. We demonstrate the programmable control of reporter genes episomally and chromosomally, with up to 25-fold more activity than seen with the EF1alpha promoter, in multiple cell types. We use these circuits to program the secretion of human monoclonal antibodies and to control T-cell effector function marked by interferon-gamma production. Antibody titers and interferon-gamma concentrations significantly correlate with synthetic promoter strengths, providing a platform for programming gene expression and cellular function in diverse applications.
Journal: Nature Communications, 2022 Oct 18 DOI: 10.1038/s41467-022-33287-9 PMID: 36257931.0 PMCID: PMC9579178
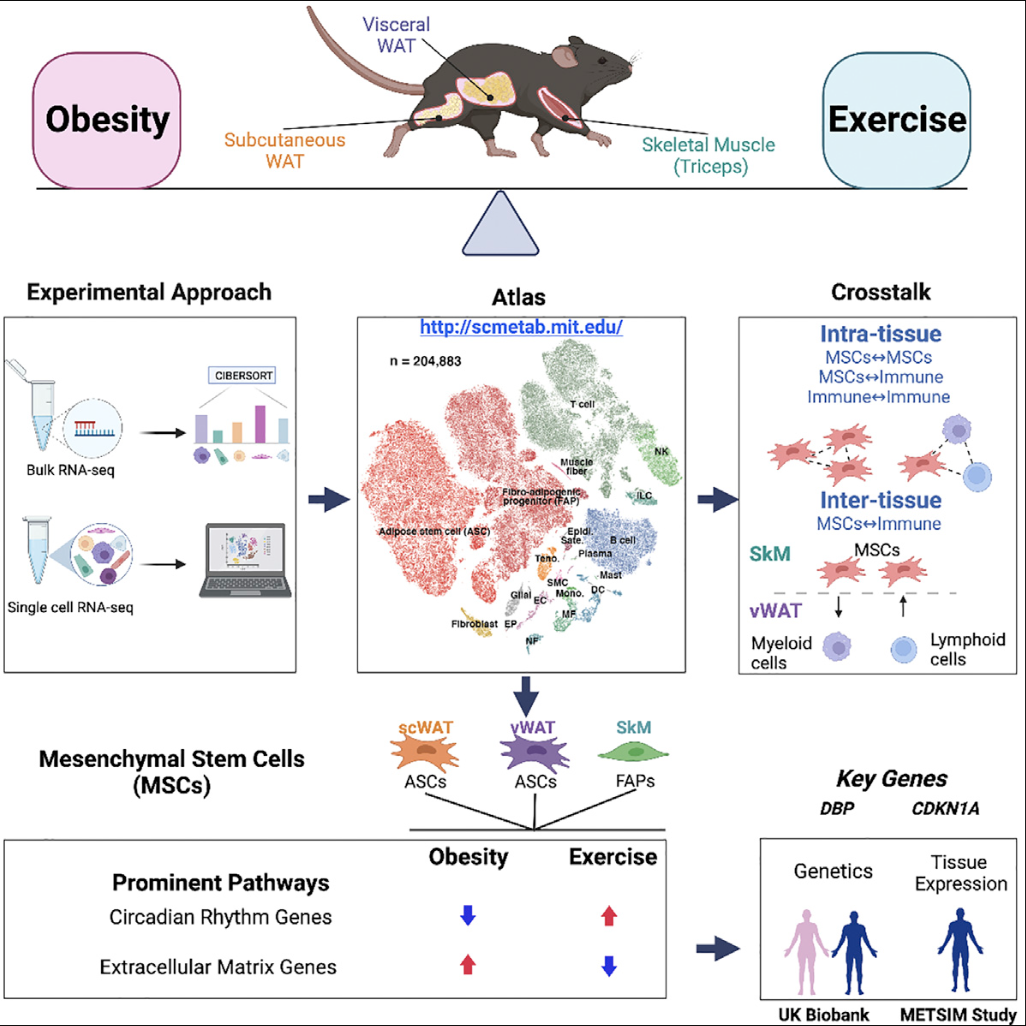
293. Single-cell dissection of the obesity-exercise axis in adipose-muscle tissues implies a critical role for mesenchymal stem cells.
Yang, Vamvini, Nigro, Ho, Galani, Alvarez, Tanigawa, Renfro, Carbone, Laakso, Agudelo, Pajukanta, Hirshman, Middelbeek, Grove, Goodyear, Kellis
Exercise training is critical for the prevention and treatment of obesity, but its underlying mechanisms remain incompletely understood given the challenge of profiling heterogeneous effects across multiple tissues and cell types. Here, we address this challenge and opposing effects of exercise and high-fat diet (HFD)-induced obesity at single-cell resolution in subcutaneous and visceral white adipose tissue and skeletal muscle in mice with diet and exercise training interventions. We identify a prominent role of mesenchymal stem cells (MSCs) in obesity and exercise-induced tissue adaptation. Among the pathways regulated by exercise and HFD in MSCs across the three tissues, extracellular matrix remodeling and circadian rhythm are the most prominent. Inferred cell-cell interactions implicate within- and multi-tissue crosstalk centered around MSCs. Overall, our work reveals the intricacies and diversity of multi-tissue molecular responses to exercise and obesity and uncovers a previously underappreciated role of MSCs in tissue-specific and multi-tissue beneficial effects of exercise.
Journal: Cell Metabolism, 2022 Oct 4 DOI: 10.1016/j.cmet.2022.09.004 PMID: 36198295.0 PMCID: PMC9558082
292. Neurons burdened by DNA double-strand breaks incite microglia activation through antiviral-like signaling in neurodegeneration.
Welch, Boix, Schmauch, Davila-Velderrain, Victor, Dileep, Bozzelli, Su, Cheng, Lee, Leary, Pfenning, Kellis, Tsai
DNA double-strand breaks (DSBs) are linked to neurodegeneration and senescence. However, it is not clear how DSB-bearing neurons influence neuroinflammation associated with neurodegeneration. Here, we characterize DSB-bearing neurons from the CK-p25 mouse model of neurodegeneration using single-nucleus, bulk, and spatial transcriptomic techniques. DSB-bearing neurons enter a late-stage DNA damage response marked by nuclear factor kappaB (NFkappaB)-activated senescent and antiviral immune pathways. In humans, Alzheimer's disease pathology is closely associated with immune activation in excitatory neurons. Spatial transcriptomics reveal that regions of CK-p25 brain tissue dense with DSB-bearing neurons harbor signatures of inflammatory microglia, which is ameliorated by NFkappaB knockdown in neurons. Inhibition of NFkappaB in DSB-bearing neurons also reduces microglia activation in organotypic mouse brain slice culture. In conclusion, DSBs activate immune pathways in neurons, which in turn adopt a senescence-associated secretory phenotype to elicit microglia activation. These findings highlight a previously unidentified role for neurons in the mechanism of disease-associated neuroinflammation.
Journal: Science Advances, 2022 Sep 30 DOI: 10.1126/sciadv.abo4662 PMID: 36170369.0 PMCID: PMC9519048
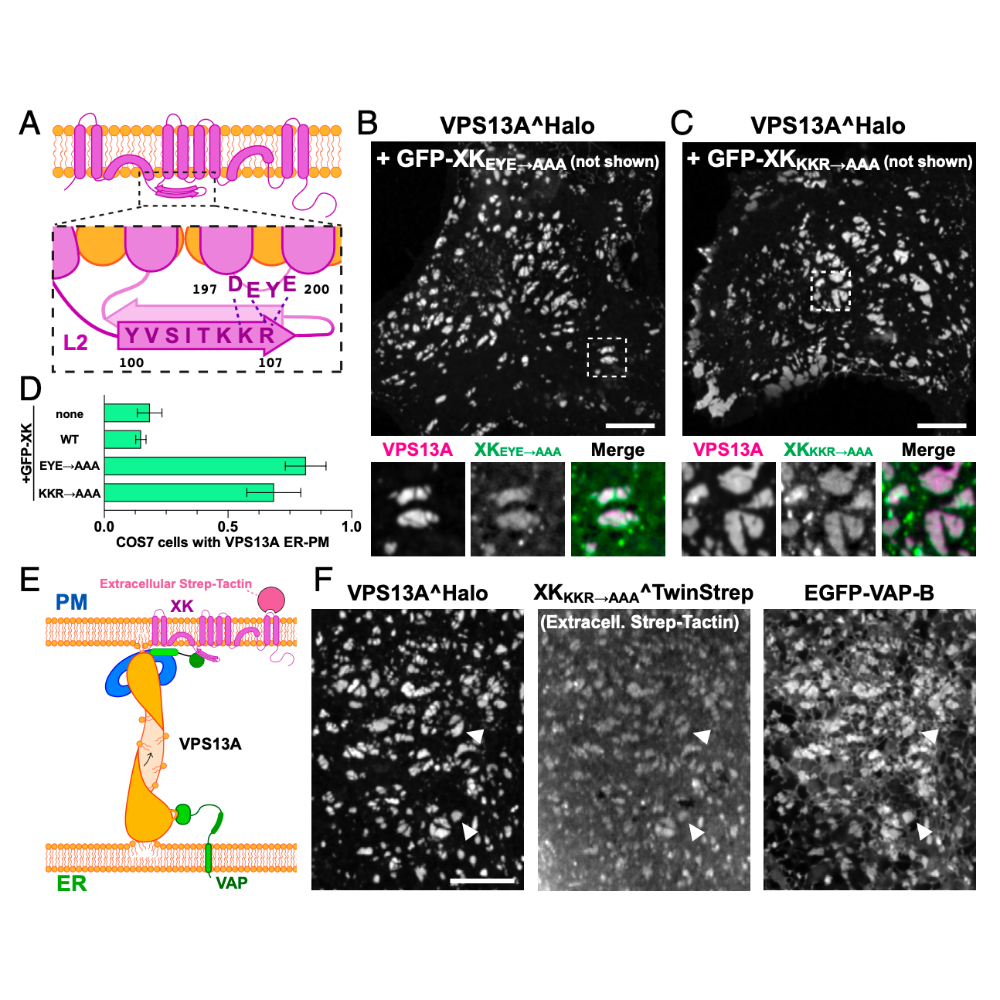
291. A partnership between the lipid scramblase XK and the lipid transfer protein VPS13A at the plasma membrane.
Guillen-Samander, Wu, Pineda, Garcia, Eisen, Leonzino, Ugur, Kellis, Heiman, De
Chorea-acanthocytosis (ChAc) and McLeod syndrome are diseases with shared clinical manifestations caused by mutations in VPS13A and XK, respectively. Key features of these conditions are the degeneration of caudate neurons and the presence of abnormally shaped erythrocytes. XK belongs to a family of plasma membrane (PM) lipid scramblases whose action results in exposure of PtdSer at the cell surface. VPS13A is an endoplasmic reticulum (ER)-anchored lipid transfer protein with a putative role in the transport of lipids at contacts of the ER with other membranes. Recently VPS13A and XK were reported to interact by still unknown mechanisms. So far, however, there is no evidence for a colocalization of the two proteins at contacts of the ER with the PM, where XK resides, as VPS13A was shown to be localized at contacts between the ER and either mitochondria or lipid droplets. Here we show that VPS13A can also localize at ER-PM contacts via the binding of its PH domain to a cytosolic loop of XK, that such interaction is regulated by an intramolecular interaction within XK, and that both VPS13A and XK are highly expressed in the caudate neurons. Binding of the PH domain of VPS13A to XK is competitive with its binding to intracellular membranes that mediate other tethering functions of VPS13A. Our findings support a model according to which VPS13A-dependent lipid transfer between the ER and the PM is coupled to lipid scrambling within the PM. They raise the possibility that defective cell surface exposure of PtdSer may be responsible for neurodegeneration.
Journal: Proceedings Of The National Academy Of Sciences Of The United States Of America, 2022 Aug 30 DOI: 10.1073/pnas.2205425119 PMID: 35994651.0 PMCID: PMC9436381
290. Standardized annotation of translated open reading frames.
Mudge, Ruiz-Orera, Prensner, Brunet, Calvet, Jungreis, Gonzalez, Magrane, Martinez, Schulz, Yang, Alba, Aspden, Baranov, Bazzini, Bruford, Martin, Calviello, Carvunis, Chen, Couso, Deutsch, Flicek, Frankish, Gerstein, Hubner, Ingolia, Kellis, Menschaert, Moritz, Ohler, Roucou, Saghatelian, Weissman, van
Journal: Nature Biotechnology, 2022 Jul DOI: 10.1038/s41587-022-01369-0 PMID: 35831657.0 PMCID: PMC9757701
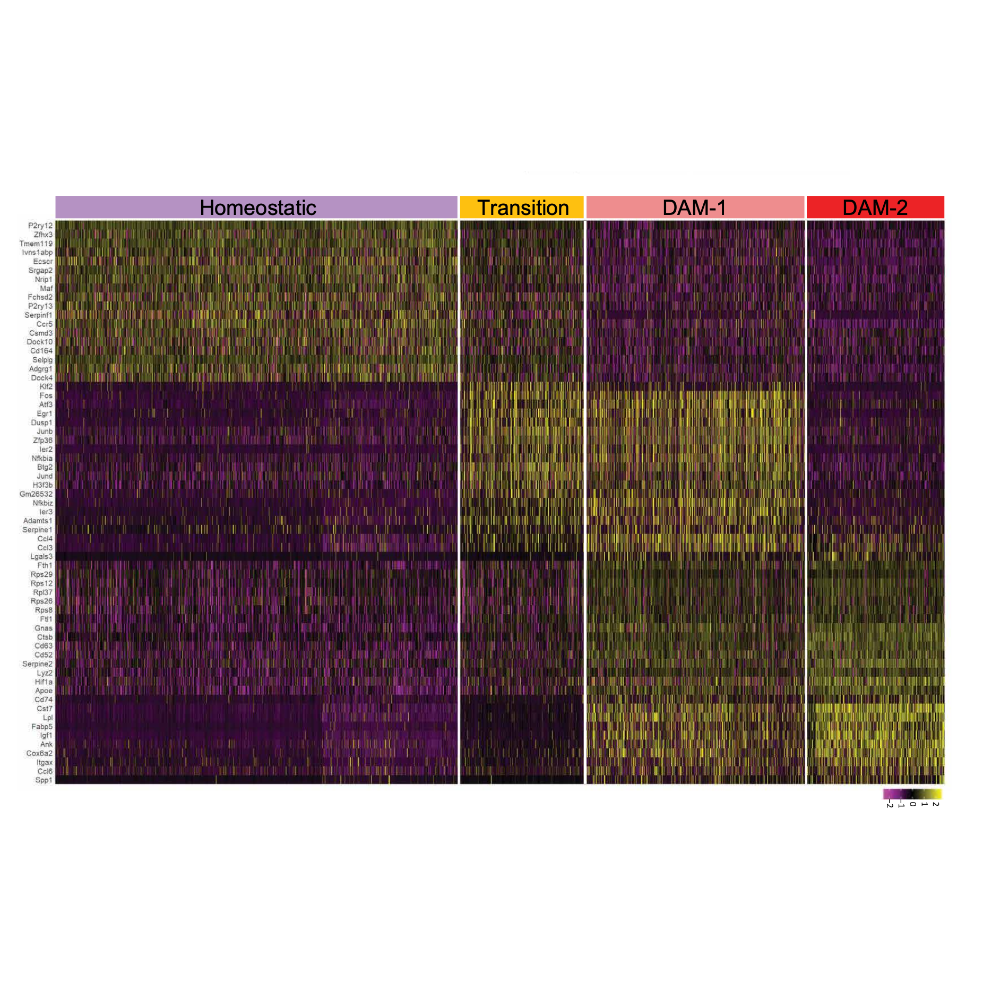
289. BACE-1 inhibition facilitates the transition from homeostatic microglia to DAM-1.
Singh, Benoit, Zhou, Das, Davila-Velderrain, Kellis, Tsai, Hu, Yan
BACE-1 is required for generating beta-amyloid (Abeta) peptides in Alzheimer's disease (AD). Here, we report that microglial BACE-1 regulates the transition of homeostatic to stage 1 disease-associated microglia (DAM-1) signature. BACE-1 deficiency elevated levels of transcription factors including Jun, Jund, Btg2, Erg1, Junb, Fos, and Fosb in the transition signature, which transition from more homeostatic to highly phagocytic DAM-1. Consistently, similar transition-state microglia in human AD brains correlated with lowered levels of BACE-1 expression. Targeted deletion of Bace-1 in adult 5xFAD mice microglia elevated these phagocytic microglia, correlated with significant reduction in amyloid plaques without synaptic toxicity. Silencing or pharmacologically inhibiting BACE-1 in cultured microglia-derived cells shows higher phagocytic function in microglia. Mechanistic exploration suggests that abolished cleavage of IL-1R2 and Toll-like receptors via BACE-1 inhibition contributes to the enhanced signaling via the PI3K and p38 MAPK kinase pathway. Together, targeted inhibition of BACE-1 in microglia may offer AD treatment.
Journal: Science Advances, 2022 Jun 17 DOI: 10.1126/sciadv.abo1286 PMID: 35714196.0 PMCID: PMC9205595
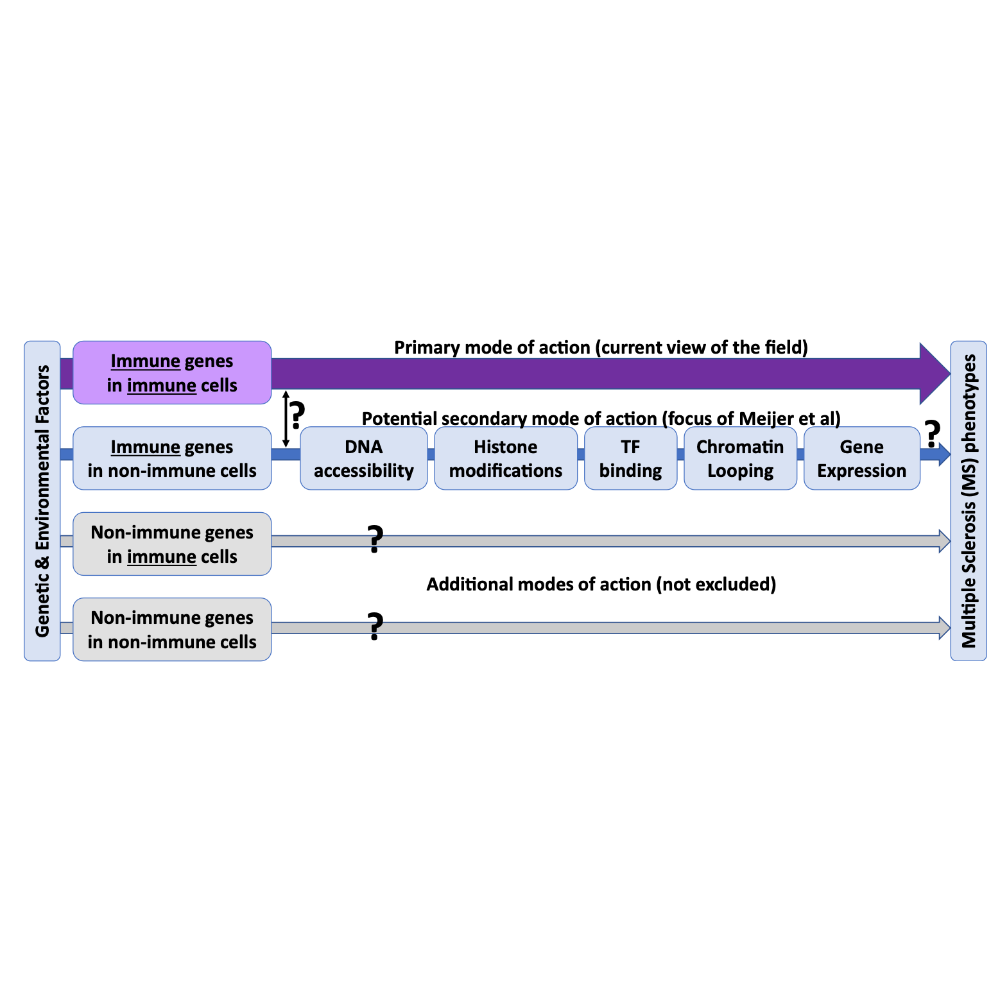
288. Immune genes outside immune cells for multiple sclerosis.
Sun, Kellis
In this issue of Neuron, Meijer and Agirre et al. (2022) demonstrate that immune genes exhibit a primed chromatin state in healthy oligodendroglia and are transcriptionally activated in MS through a series of epigenetic activations including histone modification deposition, transcription factor binding, and chromatin reconfiguration.
Journal: Neuron, 2022 Apr 6 DOI: 10.1016/j.neuron.2022.03.017 PMID: 35390286.0
287. Publisher Correction: Evolution of enhanced innate immune evasion by SARS-CoV-2.
Thorne, Bouhaddou, Reuschl, Zuliani-Alvarez, Polacco, Pelin, Batra, Whelan, Hosmillo, Fossati, Ragazzini, Jungreis, Ummadi, Rojc, Turner, Bischof, Obernier, Braberg, Soucheray, Richards, Chen, Harjai, Memon, Hiatt, Rosales, McGovern, Jahun, Fabius, White, Goodfellow, Takeuchi, Bonfanti, Shokat, Jura, Verba, Noursadeghi, Beltrao, Kellis, Swaney, Garcia-Sastre, Jolly, Towers, Krogan
Publisher Correction
Journal: Nature, 2022 Apr DOI: 10.1038/s41586-022-04653-w PMID: 35332335.0 PMCID: PMC8944406
286. Single-cell dissection of the human brain vasculature.
Garcia, Sun, Lee, Godlewski, Mathys, Galani, Zhou, Jiang, Ng, Mantero, Tsai, Bennett, Sahin, Kellis, Heiman
Despite the importance of the cerebrovasculature in maintaining normal brain physiology and in understanding neurodegeneration and drug delivery to the central nervous system(1), human cerebrovascular cells remain poorly characterized owing to their sparsity and dispersion. Here we perform single-cell characterization of the human cerebrovasculature using both ex vivo fresh tissue experimental enrichment and post mortem in silico sorting of human cortical tissue samples. We capture 16,681 cerebrovascular nuclei across 11 subtypes, including endothelial cells, mural cells and three distinct subtypes of perivascular fibroblast along the vasculature. We uncover human-specific expression patterns along the arteriovenous axis and determine previously uncharacterized cell-type-specific markers. We use these human-specific signatures to study changes in 3,945 cerebrovascular cells from patients with Huntington's disease, which reveal activation of innate immune signalling in vascular and glial cell types and a concomitant reduction in the levels of proteins critical for maintenance of blood-brain barrier integrity. Finally, our study provides a comprehensive molecular atlas of the human cerebrovasculature to guide future biological and therapeutic studies.
Journal: Nature, 2022 Mar DOI: 10.1038/s41586-022-04521-7 PMID: 35158371.0 PMCID: PMC9680899
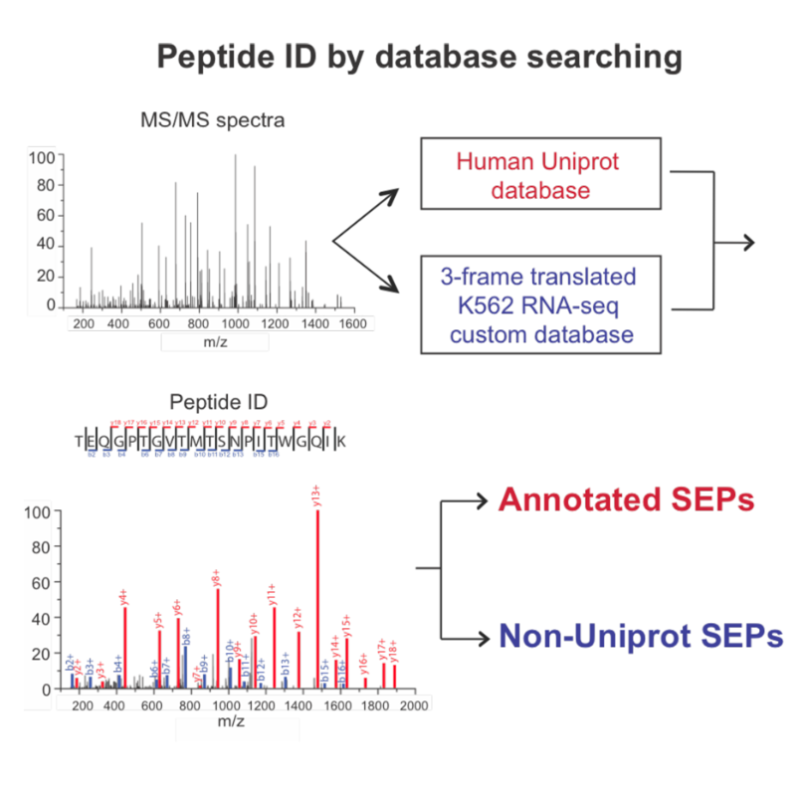
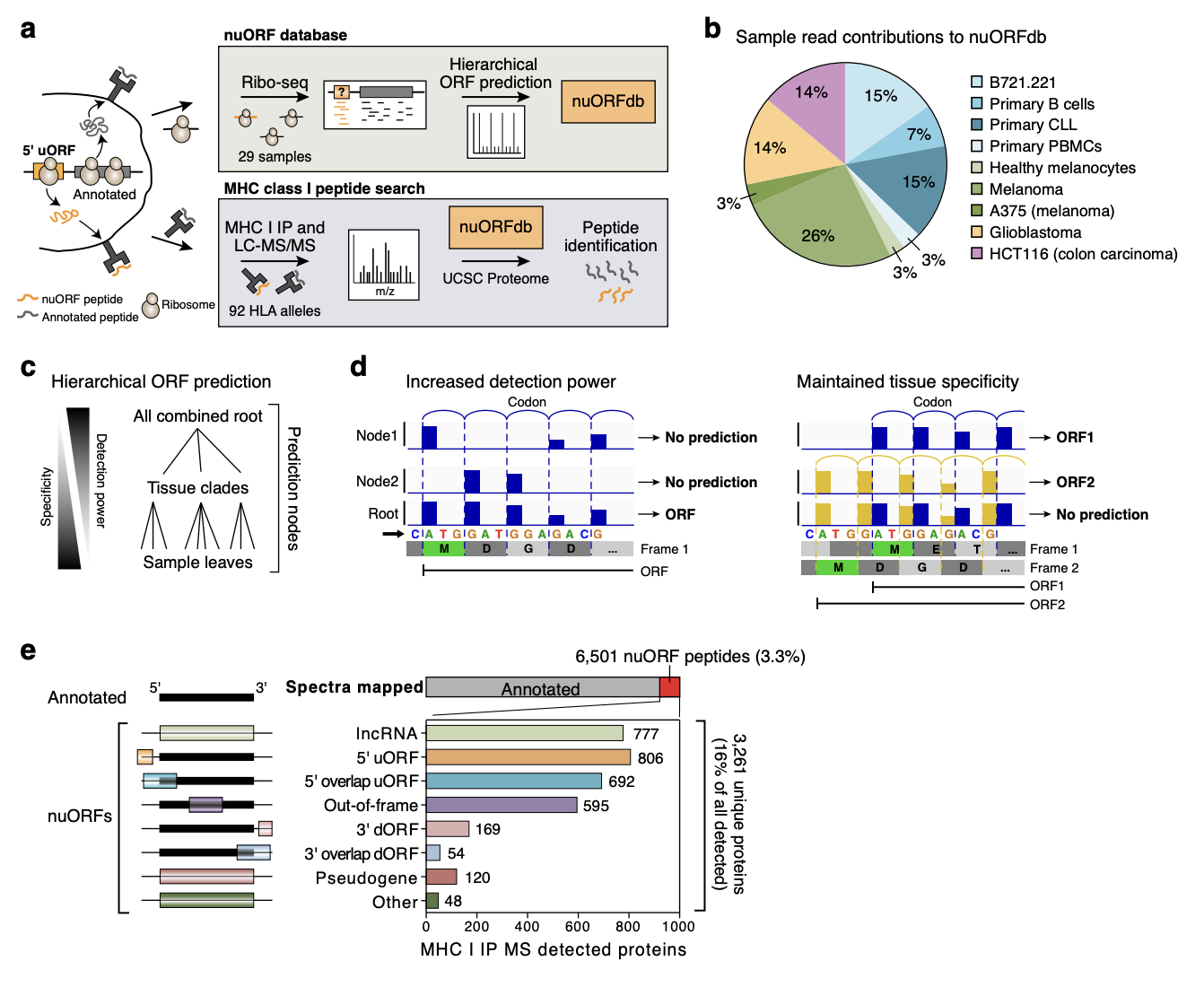
285. Unannotated proteins expand the MHC-I-restricted immunopeptidome in cancer.
Ouspenskaia, Law, Clauser, Klaeger, Sarkizova, Aguet, Li, Christian, Knisbacher, Le, Hartigan, Keshishian, Apffel, Oliveira, Zhang, Chen, Chow, Ji, Jungreis, Shukla, Justesen, Bachireddy, Kellis, Getz, Hacohen, Keskin, Carr, Wu, Regev
Tumor-associated epitopes presented on MHC-I that can activate the immune system against cancer cells are typically identified from annotated protein-coding regions of the genome, but whether peptides originating from novel or unannotated open reading frames (nuORFs) can contribute to antitumor immune responses remains unclear. Here we show that peptides originating from nuORFs detected by ribosome profiling of malignant and healthy samples can be displayed on MHC-I of cancer cells, acting as additional sources of cancer antigens. We constructed a high-confidence database of translated nuORFs across tissues (nuORFdb) and used it to detect 3,555 translated nuORFs from MHC-I immunopeptidome mass spectrometry analysis, including peptides that result from somatic mutations in nuORFs of cancer samples as well as tumor-specific nuORFs translated in melanoma, chronic lymphocytic leukemia and glioblastoma. NuORFs are an unexplored pool of MHC-I-presented, tumor-specific peptides with potential as immunotherapy targets.
Journal: Nature Biotechnology, 2022 Feb DOI: 10.1038/s41587-021-01021-3 PMID: 34663921.0 PMCID: PMC10198624
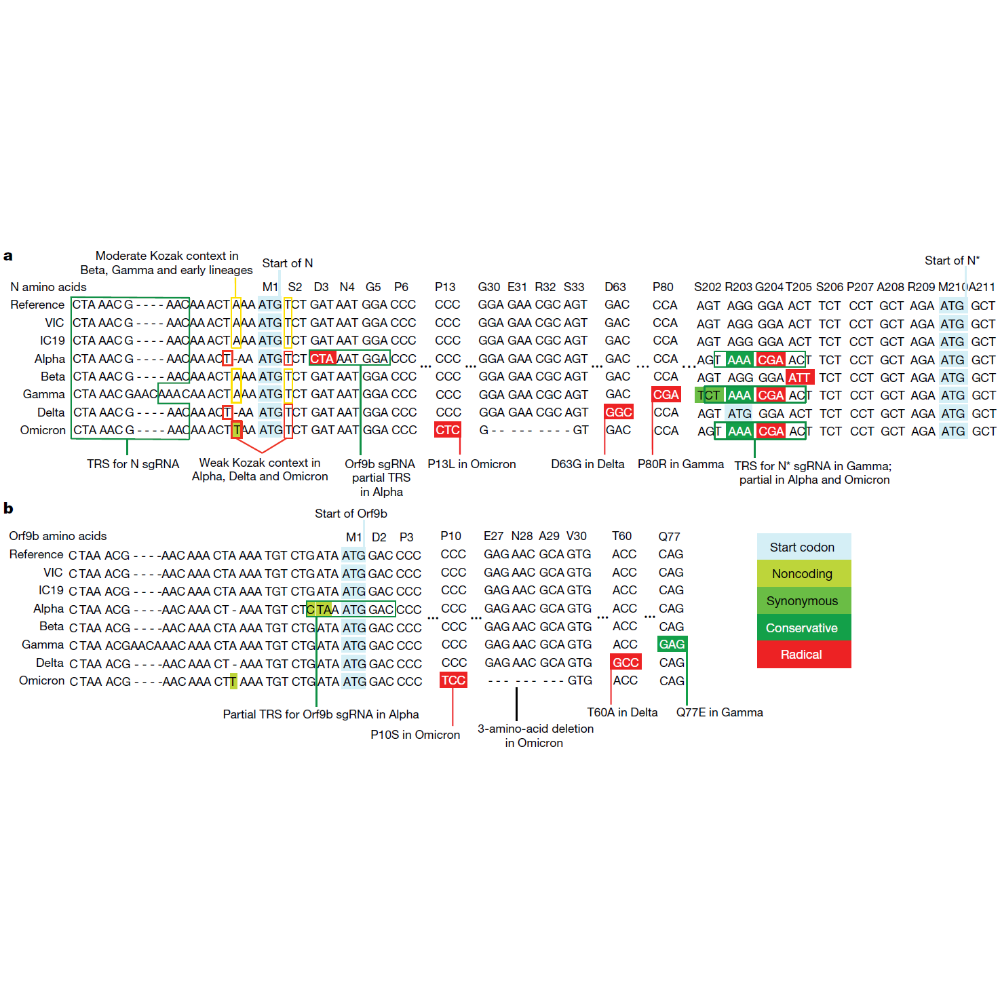
284. Evolution of enhanced innate immune evasion by SARS-CoV-2.
Thorne, Bouhaddou, Reuschl, Zuliani-Alvarez, Polacco, Pelin, Batra, Whelan, Hosmillo, Fossati, Ragazzini, Jungreis, Ummadi, Rojc, Turner, Bischof, Obernier, Braberg, Soucheray, Richards, Chen, Harjai, Memon, Hiatt, Rosales, McGovern, Jahun, Fabius, White, Goodfellow, Takeuchi, Bonfanti, Shokat, Jura, Verba, Noursadeghi, Beltrao, Kellis, Swaney, Garcia-Sastre, Jolly, Towers, Krogan
The emergence of SARS-CoV-2 variants of concern suggests viral adaptation to enhance human-to-human transmission(1,2). Although much effort has focused on the characterization of changes in the spike protein in variants of concern, mutations outside of spike are likely to contribute to adaptation. Here, using unbiased abundance proteomics, phosphoproteomics, RNA sequencing and viral replication assays, we show that isolates of the Alpha (B.1.1.7) variant(3) suppress innate immune responses in airway epithelial cells more effectively than first-wave isolates. We found that the Alpha variant has markedly increased subgenomic RNA and protein levels of the nucleocapsid protein (N), Orf9b and Orf6-all known innate immune antagonists. Expression of Orf9b alone suppressed the innate immune response through interaction with TOM70, a mitochondrial protein that is required for activation of the RNA-sensing adaptor MAVS. Moreover, the activity of Orf9b and its association with TOM70 was regulated by phosphorylation. We propose that more effective innate immune suppression, through enhanced expression of specific viral antagonist proteins, increases the likelihood of successful transmission of the Alpha variant, and may increase in vivo replication and duration of infection(4). The importance of mutations outside the spike coding region in the adaptation of SARS-CoV-2 to humans is underscored by the observation that similar mutations exist in the N and Orf9b regulatory regions of the Delta and Omicron variants.
Journal: Nature, 2022 Feb DOI: 10.1038/s41586-021-04352-y PMID: 34942634.0 PMCID: PMC8850198
283. MEF2 is a key regulator of cognitive potential and confers resilience to neurodegeneration.
Barker, Raju, Milman, Wang, Davila-Velderrain, Gunter-Rahman, Parro, Bozzelli, Abdurrob, Abdelaal, Bennett, Kellis, Tsai
Recent increases in human longevity have been accompanied by a rise in the incidence of dementia, highlighting the need to preserve cognitive function in an aging population. A small percentage of individuals with pathological hallmarks of neurodegenerative disease are able to maintain normal cognition. Although the molecular mechanisms that govern this neuroprotection remain unknown, individuals that exhibit cognitive resilience (CgR) represent a unique source of therapeutic insight. For both humans and animal models, living in an enriched, cognitively stimulating environment is the most effective known inducer of CgR. To understand potential drivers of this phenomenon, we began by profiling the molecular changes that arise from environmental enrichment in mice, which led to the identification of MEF2 transcription factors (TFs). We next turned to repositories of human clinical and brain transcriptomic data, where we found that the MEF2 transcriptional network was overrepresented among genes that are most predictive of end-stage cognition. Through single-nucleus RNA sequencing of cortical tissue from resilient and nonresilient individuals, we further confirmed up-regulation of MEF2C in resilient individuals to a subpopulation of excitatory neurons. Last, to determine the causal impact of MEF2 on cognition in the context of neurodegeneration, we overexpressed Mef2a/c in the PS19 mouse model of tauopathy and found that this was sufficient to improve cognitive flexibility and reduce hyperexcitability. Overall, our findings reveal a previously unappreciated role for MEF2 TFs in promoting CgR, highlighting their potential as biomarkers or therapeutic targets for neurodegeneration and healthy aging.
Journal: Science Translational Medicine, 2021 Nov 3 DOI: 10.1126/scitranslmed.abd7695 PMID: 34731014.0 PMCID: PMC9258338
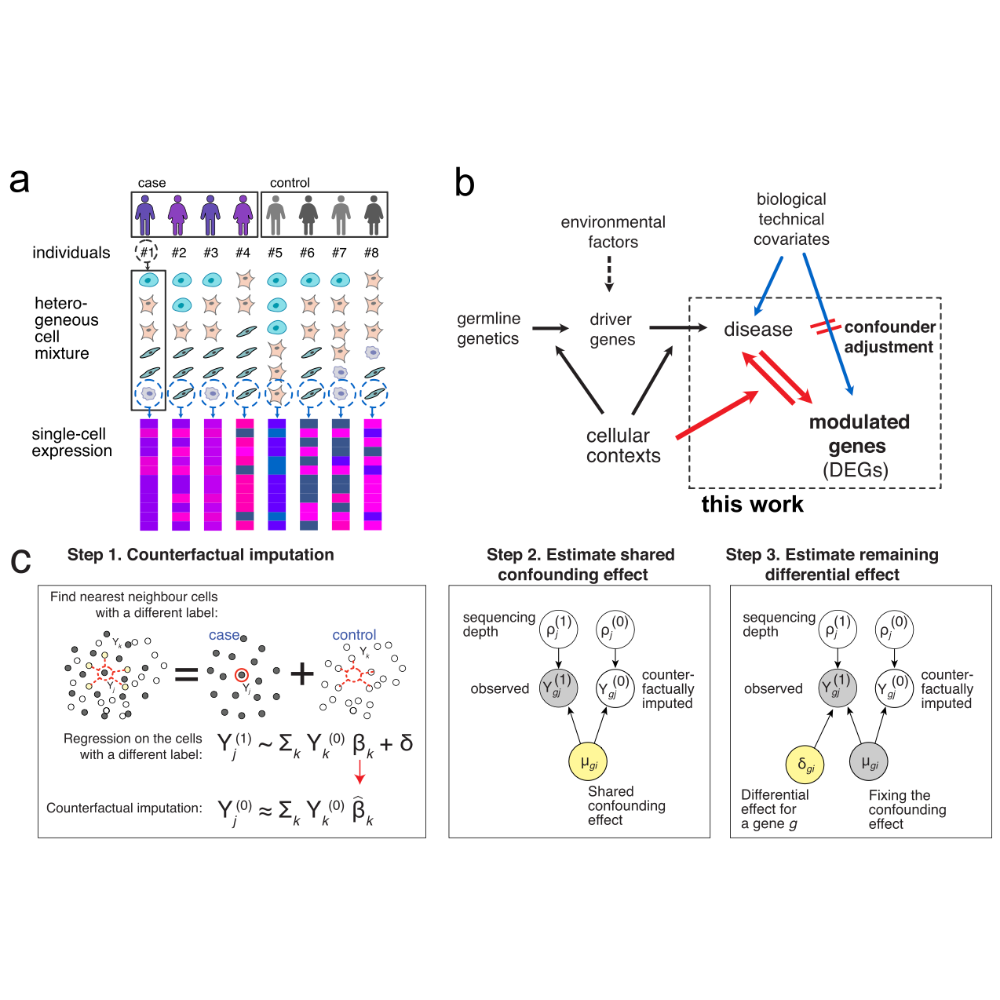
282. CoCoA-diff: counterfactual inference for single-cell gene expression analysis.
Park, Kellis
Finding a causal gene is a fundamental problem in genomic medicine. We present a causal inference framework, CoCoA-diff, that prioritizes disease genes by adjusting confounders without prior knowledge of control variables in single-cell RNA-seq data. We demonstrate that our method substantially improves statistical power in simulations and real-world data analysis of 70k brain cells collected for dissecting Alzheimer's disease. We identify 215 differentially regulated causal genes in various cell types, including highly relevant genes with a proper cell type context. Genes found in different types enrich distinctive pathways, implicating the importance of cell types in understanding multifaceted disease mechanisms.
Journal: Genome Biology, 2021 Aug 17 DOI: 10.1186/s13059-021-02438-4 PMID: 34404460.0 PMCID: PMC8369635
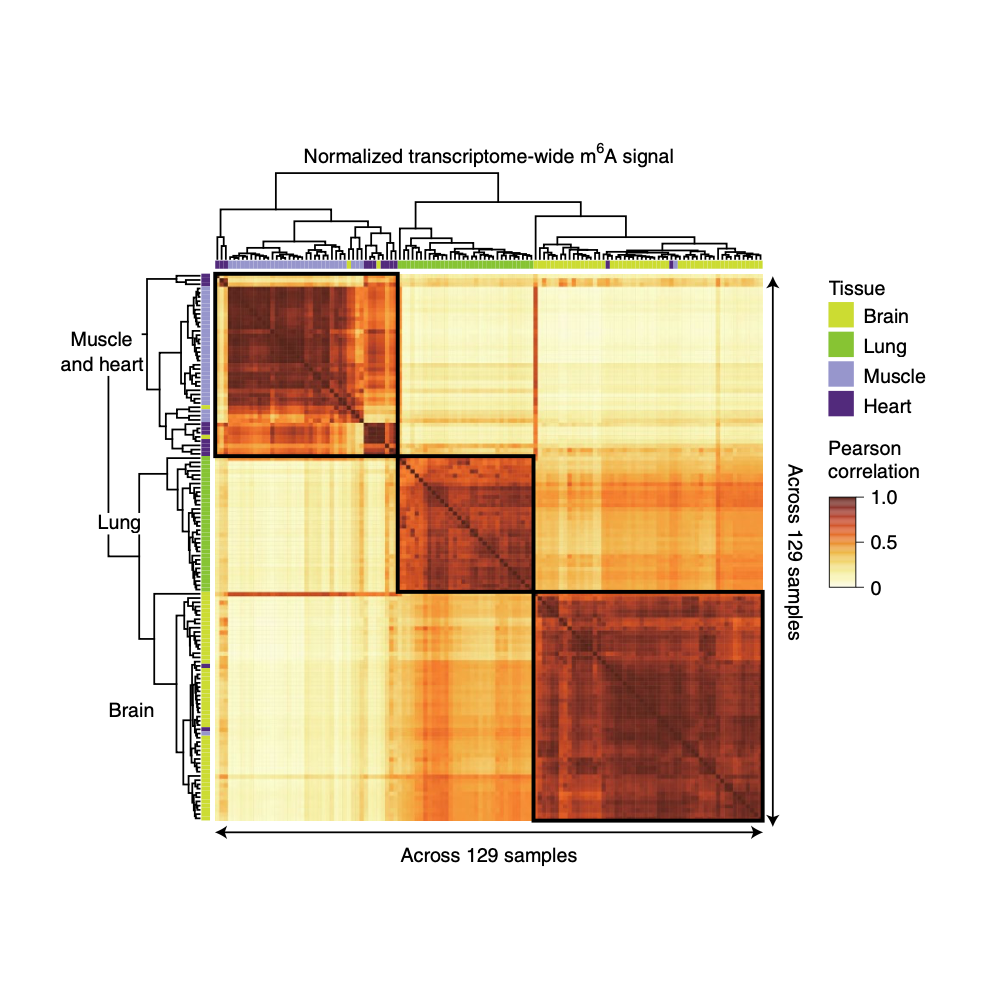
281. Genetic drivers of m(6)A methylation in human brain, lung, heart and muscle.
Xiong, Hou, Park, Molinie, Gregory, Kellis
The most prevalent post-transcriptional mRNA modification, N(6)-methyladenosine (m(6)A), plays diverse RNA-regulatory roles, but its genetic control in human tissues remains uncharted. Here we report 129 transcriptome-wide m(6)A profiles, covering 91 individuals and 4 tissues (brain, lung, muscle and heart) from GTEx/eGTEx. We integrate these with interindividual genetic and expression variation, revealing 8,843 tissue-specific and 469 tissue-shared m(6)A quantitative trait loci (QTLs), which are modestly enriched in, but mostly orthogonal to, expression QTLs. We integrate m(6)A QTLs with disease genetics, identifying 184 GWAS-colocalized m(6)A QTL, including brain m(6)A QTLs underlying neuroticism, depression, schizophrenia and anxiety; lung m(6)A QTLs underlying expiratory flow and asthma; and muscle/heart m(6)A QTLs underlying coronary artery disease. Last, we predict novel m(6)A regulators that show preferential binding in m(6)A QTLs, protein interactions with known m(6)A regulators and expression correlation with the m(6)A levels of their targets. Our results provide important insights and resources for understanding both cis and trans regulation of epitranscriptomic modifications, their interindividual variation and their roles in human disease.
Journal: Nature Genetics, 2021 Aug DOI: 10.1038/s41588-021-00890-3 PMID: 34211177.0 PMCID: PMC9112289
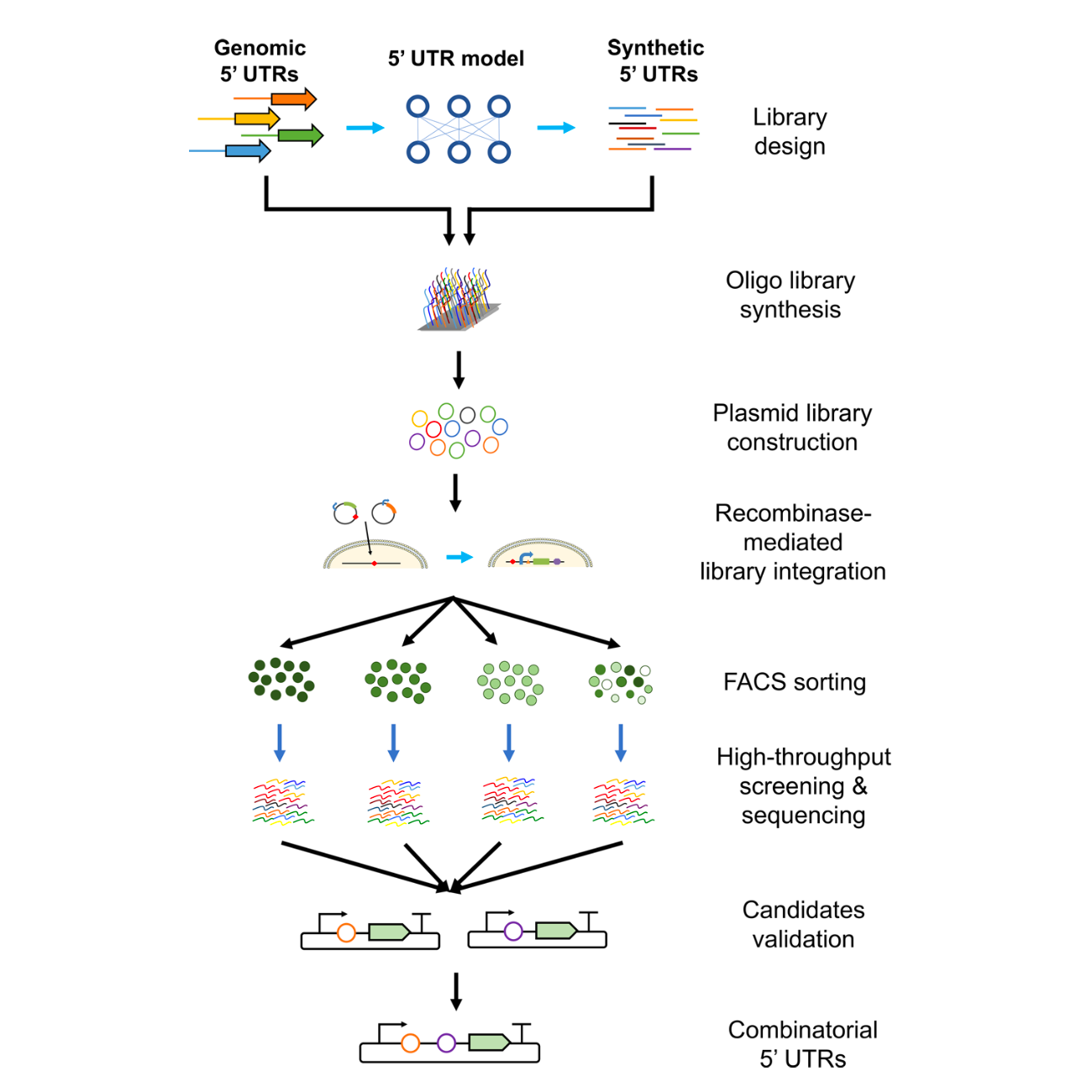
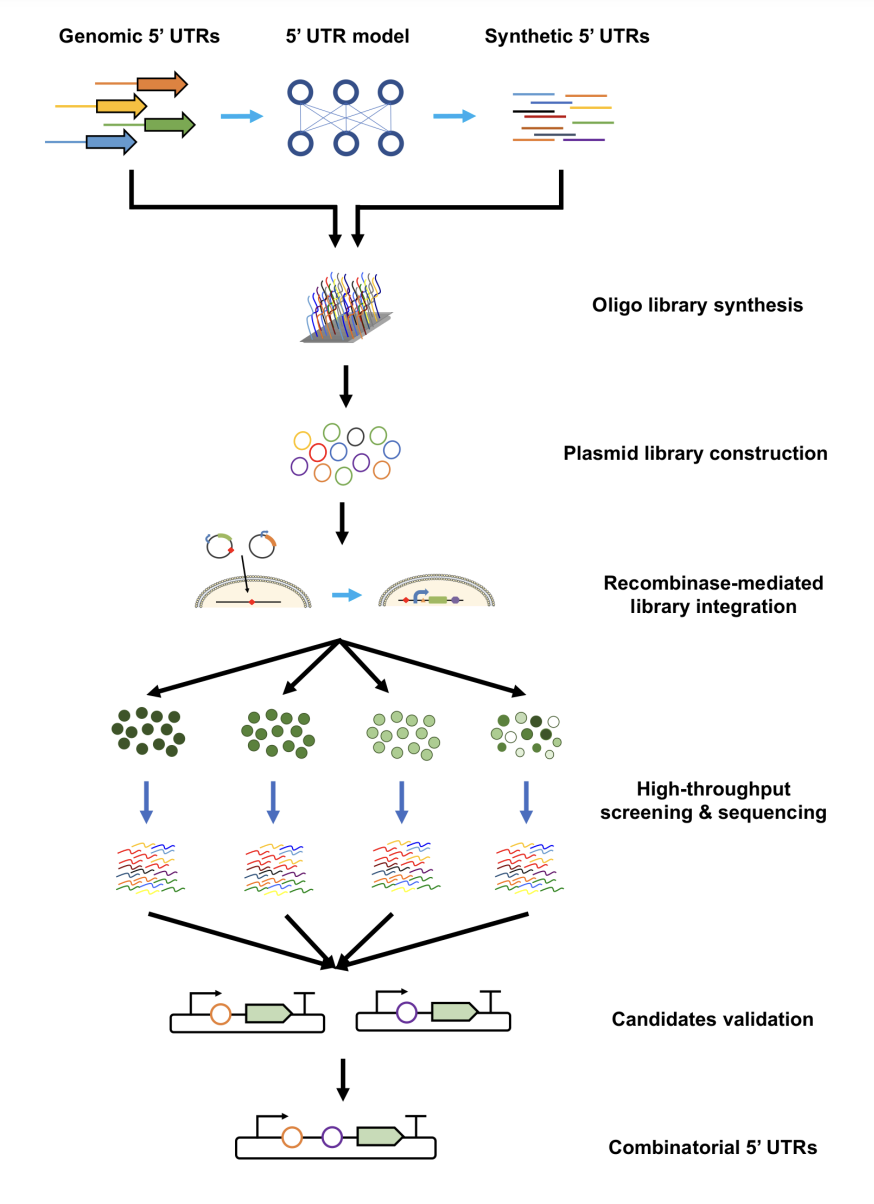
280. High-throughput 5' UTR engineering for enhanced protein production in non-viral gene therapies.
Cao, Novoa, Zhang, Chen, Liu, Choi, Wong, Wehrspaun, Kellis, Lu
Despite significant clinical progress in cell and gene therapies, maximizing protein expression in order to enhance potency remains a major technical challenge. Here, we develop a high-throughput strategy to design, screen, and optimize 5' UTRs that enhance protein expression from a strong human cytomegalovirus (CMV) promoter. We first identify naturally occurring 5' UTRs with high translation efficiencies and use this information with in silico genetic algorithms to generate synthetic 5' UTRs. A total of ~12,000 5' UTRs are then screened using a recombinase-mediated integration strategy that greatly enhances the sensitivity of high-throughput screens by eliminating copy number and position effects that limit lentiviral approaches. Using this approach, we identify three synthetic 5' UTRs that outperform commonly used non-viral gene therapy plasmids in expressing protein payloads. In summary, we demonstrate that high-throughput screening of 5' UTR libraries with recombinase-mediated integration can identify genetic elements that enhance protein expression, which should have numerous applications for engineered cell and gene therapies.
Journal: Nature Communications, 2021 Jul 6 DOI: 10.1038/s41467-021-24436-7 PMID: 34230498.0 PMCID: PMC8260622
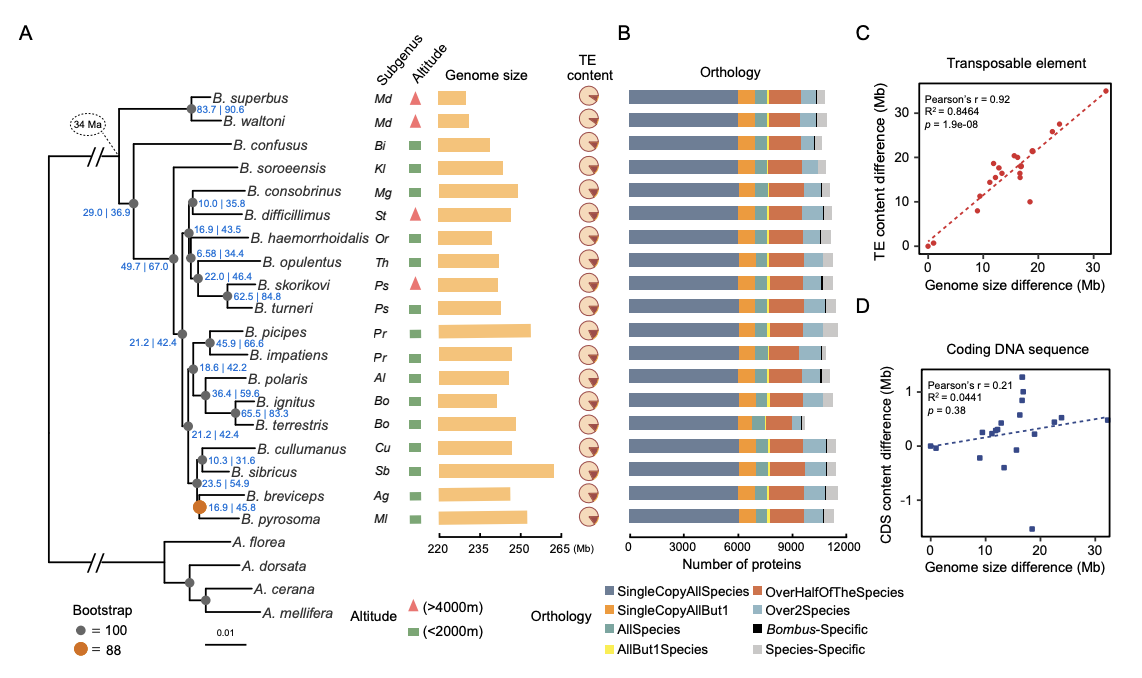
279. Erratum to: Genus-wide characterization of bumblebee genomes provides insights into their evolution and variation in ecological and behavioral traits.
Sun, Huang, Wang, Zhao, Su, Thomas, Zhao, Zhang, Jungreis, Kellis, Vicario, Sharakhov, Bondarenko, Hasselmann, Kim, Paten, Penso-Dolfin, Wang, Chang, Gao, Ma, Ma, Zhang, Zhang, Zhang, Ruzzante, Robertson, Zhu, Liu, Yang, Ding, Wang, Ma, Xu, Liang, Itgen, Mee, Cao, Zhang, Sadd, Hahn, Schaack, Barribeau, Williams, Waterhouse, Mueller
Bumblebees are a diverse group of globally important pollinators in natural ecosystems and for agricultural food production. With both eusocial and solitary life- cycle phases, and some social parasite species, they are especially interesting models to understand social evolution, behavior, and ecology. Reports of many species in decline point to pathogen transmission, habitat loss, pesticide usage, and global climate change, as interconnected causes. These threats to bumblebee diversity make our reliance on a handful of well-studied species for agricultural pollination particularly precarious. To broadly sample bumblebee genomic and phenotypic diversity, we de novo sequenced and assembled the genomes of 17 species, representing all 15 subgenera, producing the first genus-wide quantification of genetic and genomic variation potentially underlying key ecological and behavioral traits. The species phylogeny resolves subgenera relationships while incomplete lineage sorting likely drives high levels of gene tree discordance. Five chromosome-level assemblies show a stable 18-chromosome karyotype, with major rearrangements creating 25 chromosomes in social parasites. Differential transposable element activity drives changes in genome sizes, with putative domestications of repetitive sequences influencing gene coding and regulatory potential. Dynamically evolving gene families and signatures of positive selection point to genus-wide variation in processes linked to foraging, diet and metabolism, immunity and detoxification, as well as adaptations for life at high altitudes. Our study reveals how bumblebee genes and genomes have evolved across the Bombus phylogeny and identifies variations potentially linked to key ecological and behavioral traits of these important pollinators.
Journal: Molecular Biology And Evolution, 2021 Jun 25 DOI: 10.1093/molbev/msab100 PMID: 34015138.0 PMCID: PMC8233484
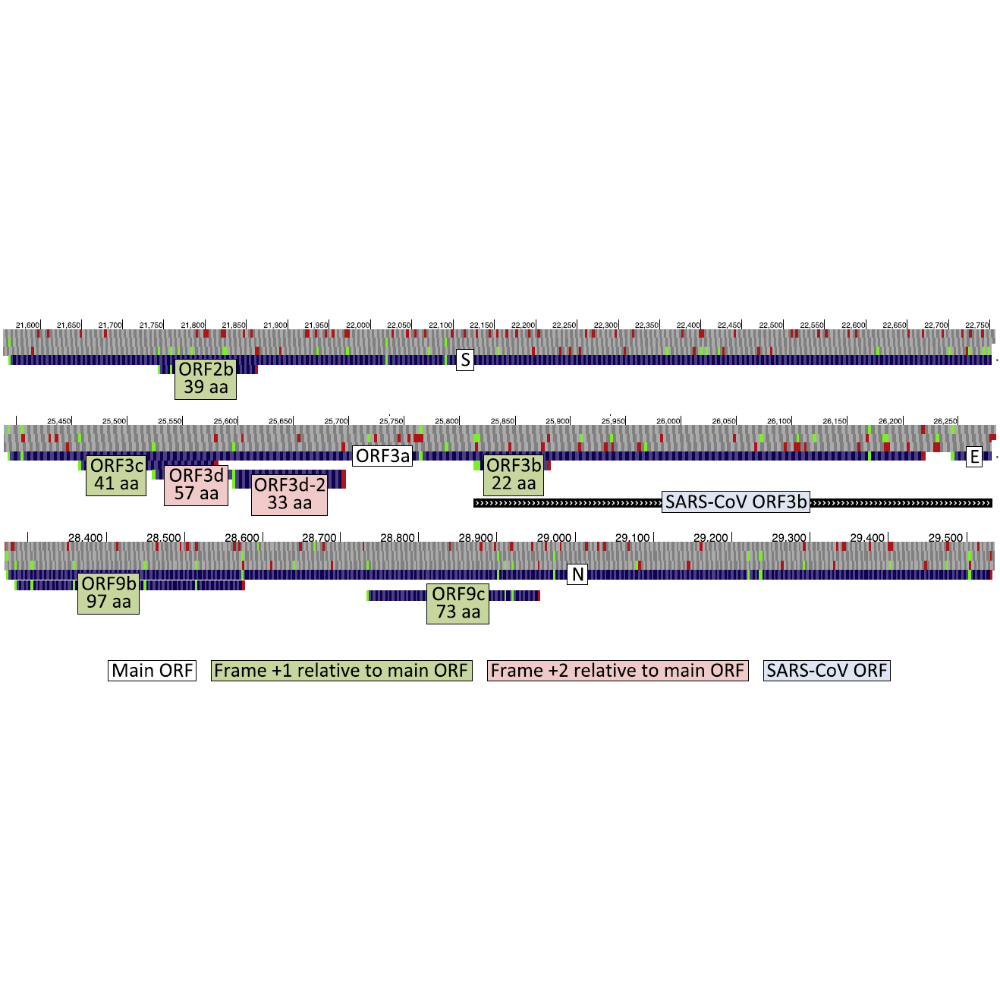
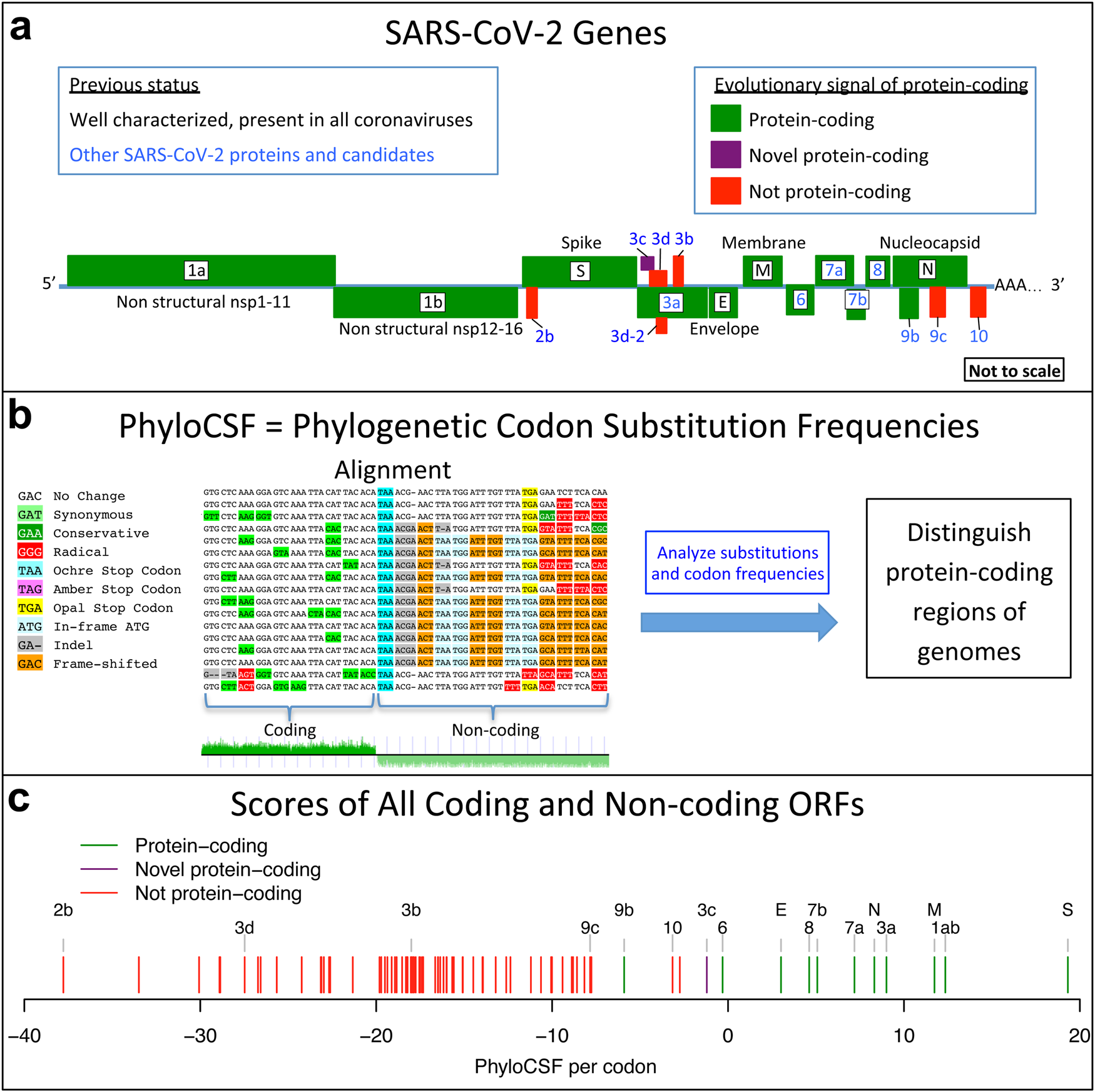
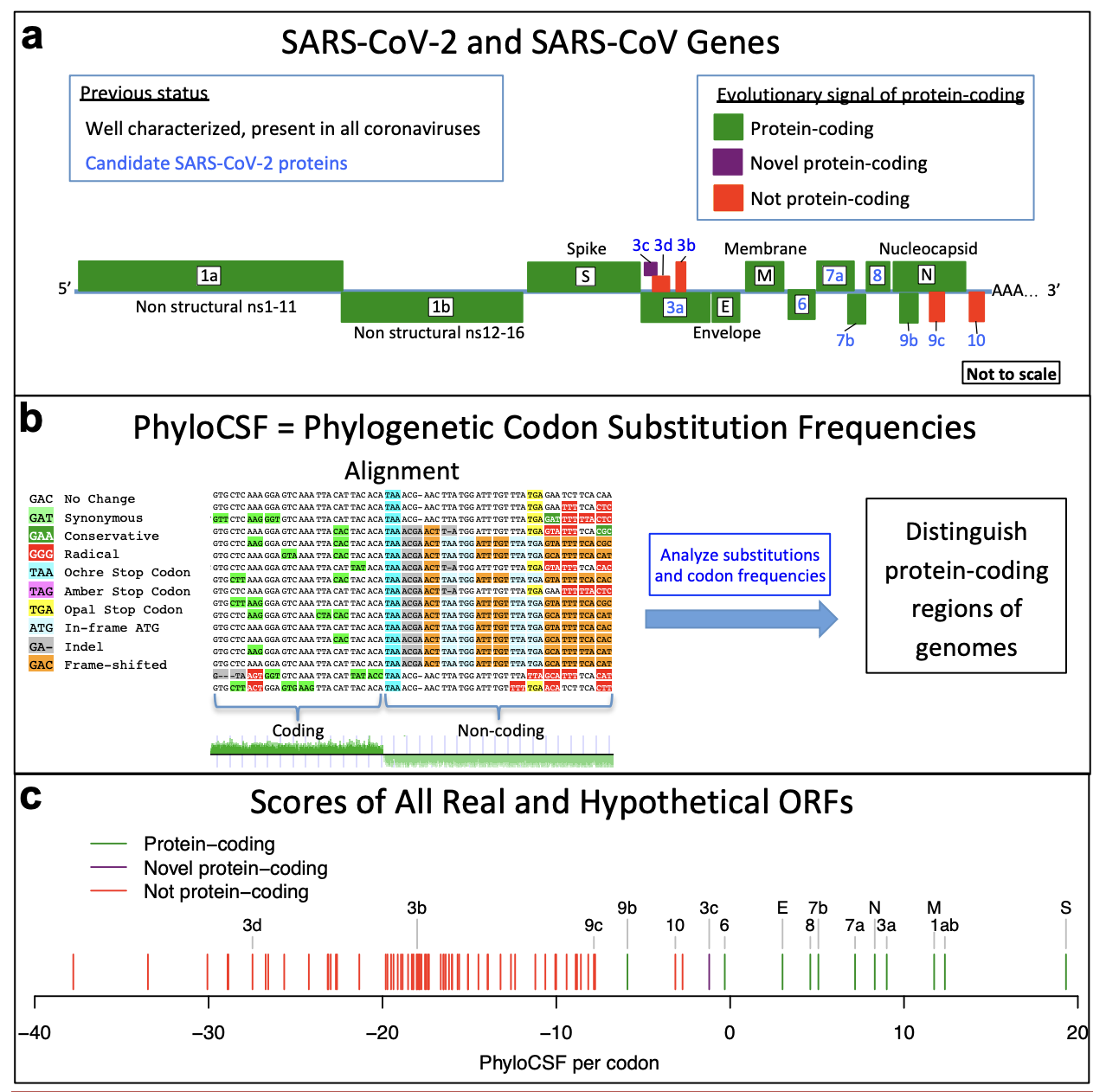
278. Conflicting and ambiguous names of overlapping ORFs in the SARS-CoV-2 genome: A homology-based resolution.
Jungreis, Nelson, Ardern, Finkel, Krogan, Sato, Ziebuhr, Stern-Ginossar, Pavesi, Firth, Gorbalenya, Kellis
At least six small alternative-frame open reading frames (ORFs) overlapping well-characterized SARS-CoV-2 genes have been hypothesized to encode accessory proteins. Researchers have used different names for the same ORF or the same name for different ORFs, resulting in erroneous homological and functional inferences. We propose standard names for these ORFs and their shorter isoforms, developed in consultation with the Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. We recommend calling the 39 codon Spike-overlapping ORF ORF2b; the 41, 57, and 22 codon ORF3a-overlapping ORFs ORF3c, ORF3d, and ORF3b; the 33 codon ORF3d isoform ORF3d-2; and the 97 and 73 codon Nucleocapsid-overlapping ORFs ORF9b and ORF9c. Finally, we document conflicting usage of the name ORF3b in 32 studies, and consequent erroneous inferences, stressing the importance of reserving identical names for homologs. We recommend that authors referring to these ORFs provide lengths and coordinates to minimize ambiguity caused by prior usage of alternative names.
Journal: Virology, 2021 Jun DOI: 10.1016/j.virol.2021.02.013 PMID: 33774510.0 PMCID: PMC7967279
277. Evolution of delayed resistance to immunotherapy in a melanoma responder.
Liu, Lin, Robitschek, Kasumova, Heyde, Shi, Kraya, Zhang, Moll, Frederick, Chen, Wang, Schapiro, Ho, Bi, Sahu, Mei, Miao, Sharova, Alvarez-Breckenridge, Stocking, Kim, Fadden, Lawrence, Hoang, Cahill, Malehmir, Nowak, Brastianos, Lian, Ruppin, Izar, Herlyn, Van, Nathanson, Flaherty, Sullivan, Kellis, Sorger, Boland
Despite initial responses(1-3), most melanoma patients develop resistance(4) to immune checkpoint blockade (ICB). To understand the evolution of resistance, we studied 37 tumor samples over 9 years from a patient with metastatic melanoma with complete clinical response to ICB followed by delayed recurrence and death. Phylogenetic analysis revealed co-evolution of seven lineages with multiple convergent, but independent resistance-associated alterations. All recurrent tumors emerged from a lineage characterized by loss of chromosome 15q, with post-treatment clones acquiring additional genomic driver events. Deconvolution of bulk RNA sequencing and highly multiplexed immunofluorescence (t-CyCIF) revealed differences in immune composition among different lineages. Imaging revealed a vasculogenic mimicry phenotype in NGFR(hi) tumor cells with high PD-L1 expression in close proximity to immune cells. Rapid autopsy demonstrated two distinct NGFR spatial patterns with high polarity and proximity to immune cells in subcutaneous tumors versus a diffuse spatial pattern in lung tumors, suggesting different roles of this neural-crest-like program in different tumor microenvironments. Broadly, this study establishes a high-resolution map of the evolutionary dynamics of resistance to ICB, characterizes a de-differentiated neural-crest tumor population in melanoma immunotherapy resistance and describes site-specific differences in tumor-immune interactions via longitudinal analysis of a patient with melanoma with an unusual clinical course.
Journal: Nature Medicine, 2021 Jun DOI: 10.1038/s41591-021-01331-8 PMID: 33941922.0 PMCID: PMC8474080
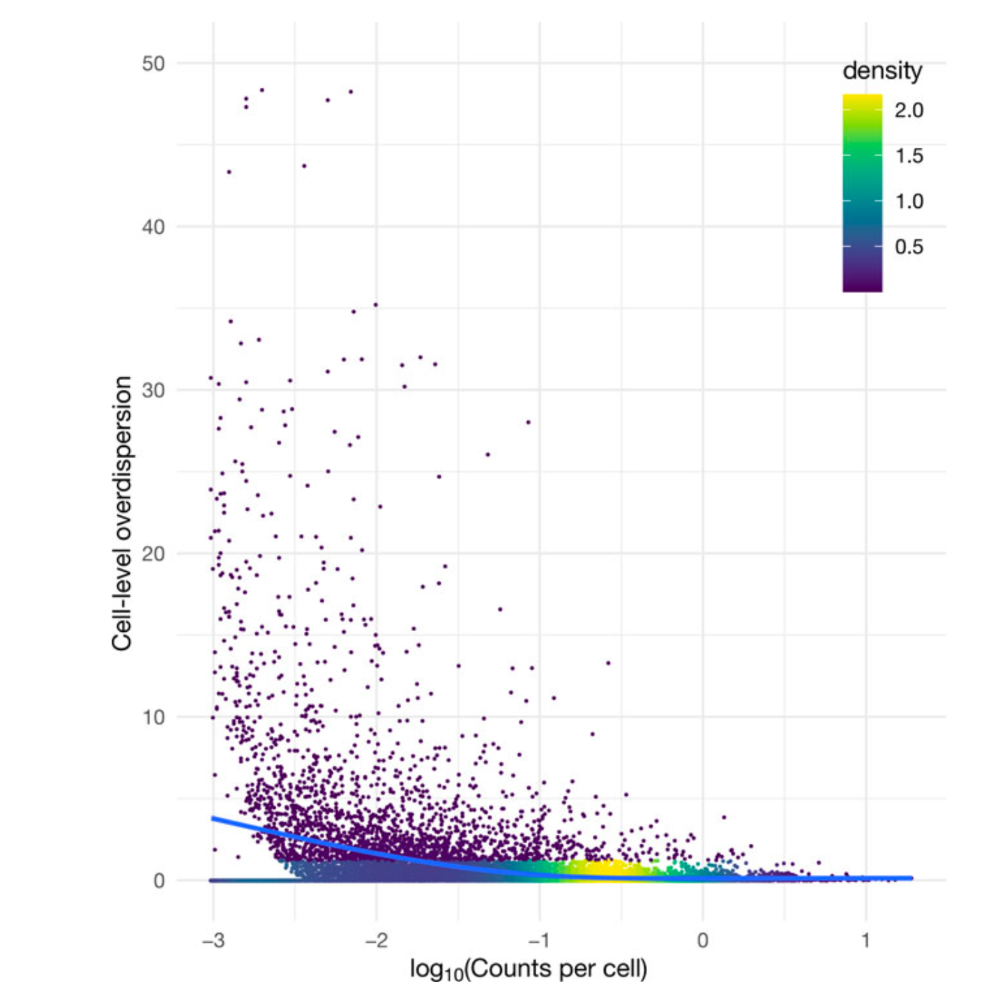
276. NEBULA is a fast negative binomial mixed model for differential or co-expression analysis of large-scale multi-subject single-cell data.
He, Davila-Velderrain, Sumida, Hafler, Kellis, Kulminski
The increasing availability of single-cell data revolutionizes the understanding of biological mechanisms at cellular resolution. For differential expression analysis in multi-subject single-cell data, negative binomial mixed models account for both subject-level and cell-level overdispersions, but are computationally demanding. Here, we propose an efficient NEgative Binomial mixed model Using a Large-sample Approximation (NEBULA). The speed gain is achieved by analytically solving high-dimensional integrals instead of using the Laplace approximation. We demonstrate that NEBULA is orders of magnitude faster than existing tools and controls false-positive errors in marker gene identification and co-expression analysis. Using NEBULA in Alzheimer's disease cohort data sets, we found that the cell-level expression of APOE correlated with that of other genetic risk factors (including CLU, CST3, TREM2, C1q, and ITM2B) in a cell-type-specific pattern and an isoform-dependent manner in microglia. NEBULA opens up a new avenue for the broad application of mixed models to large-scale multi-subject single-cell data.
Journal: Communications Biology, 2021 May 26 DOI: 10.1038/s42003-021-02146-6 PMID: 34040149.0 PMCID: PMC8155058
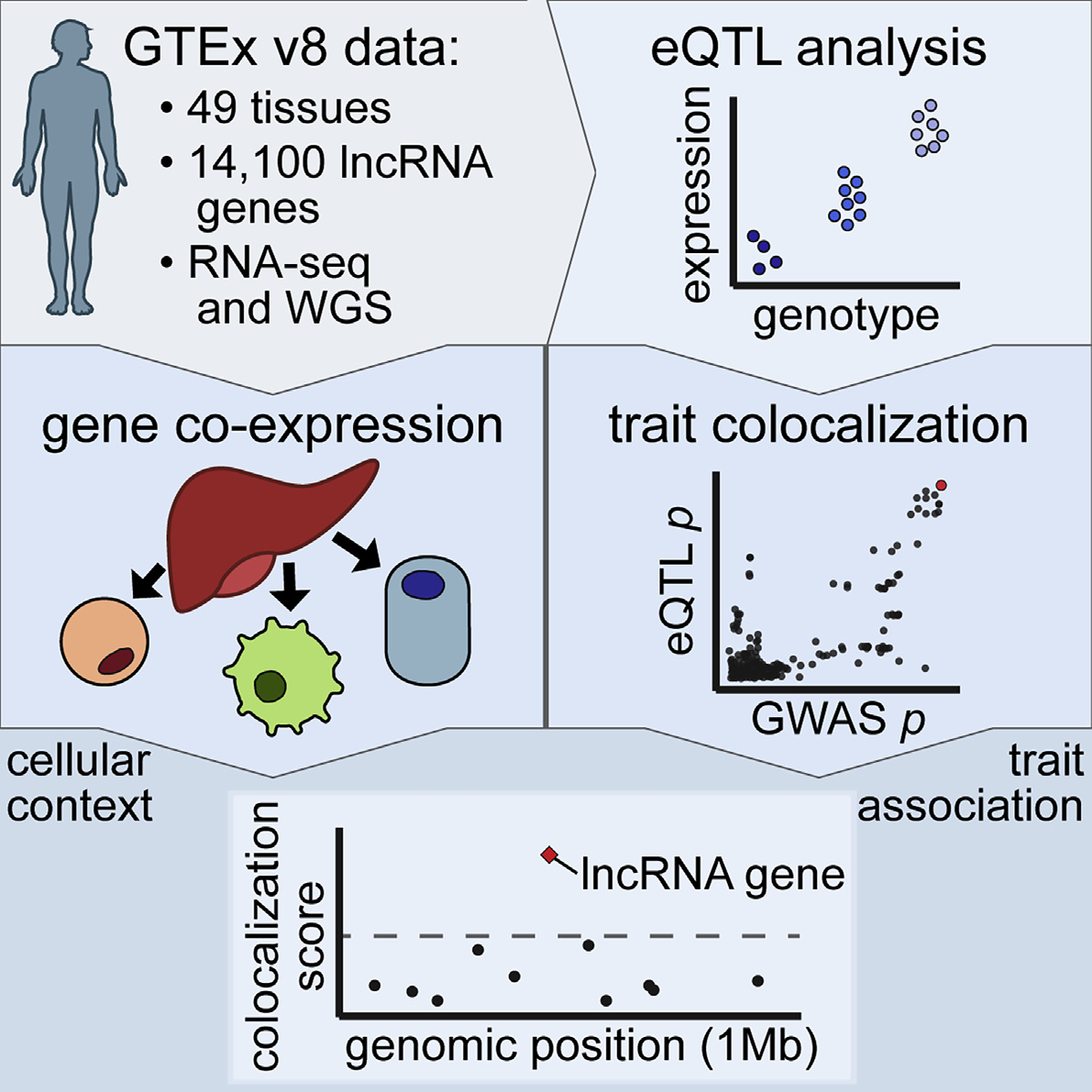
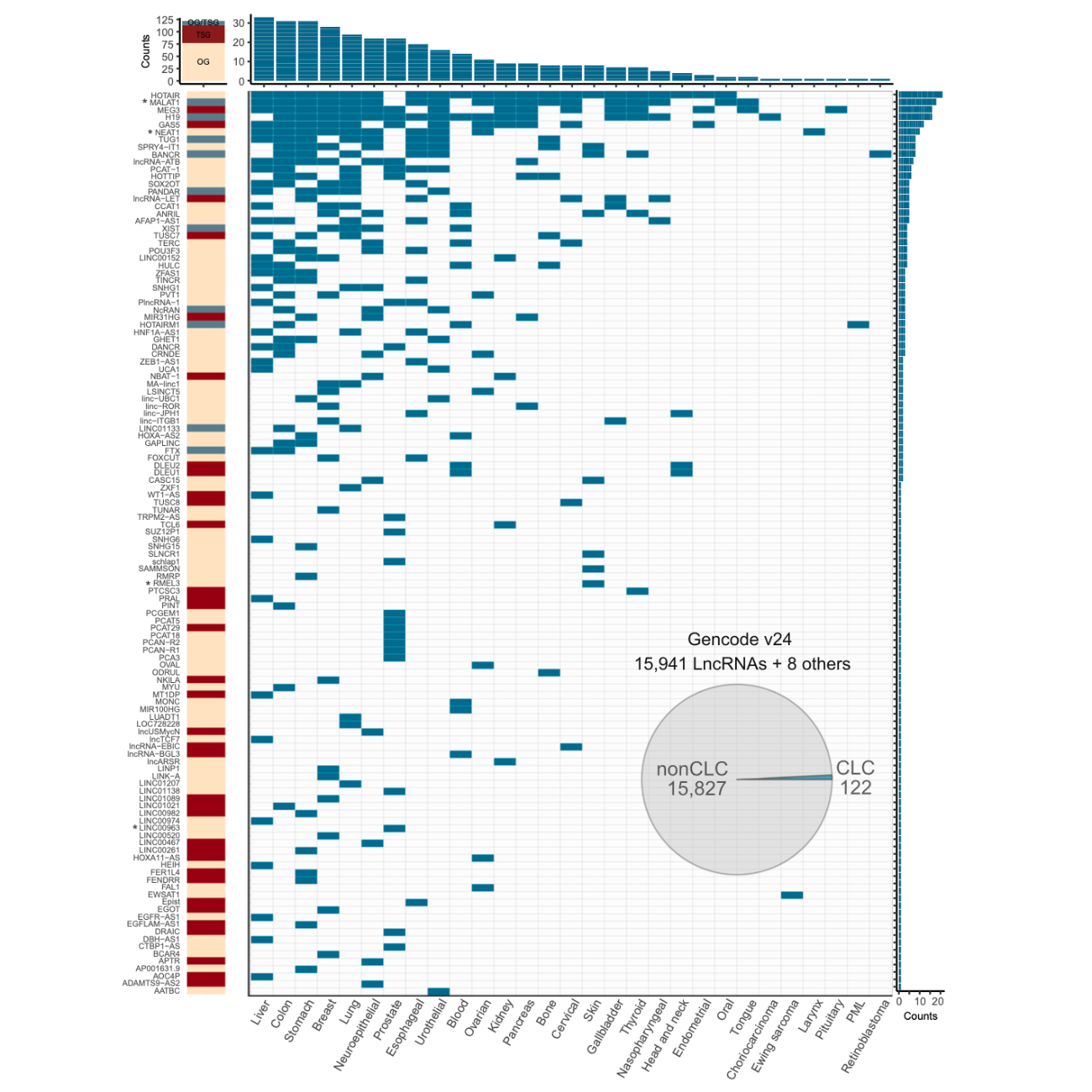
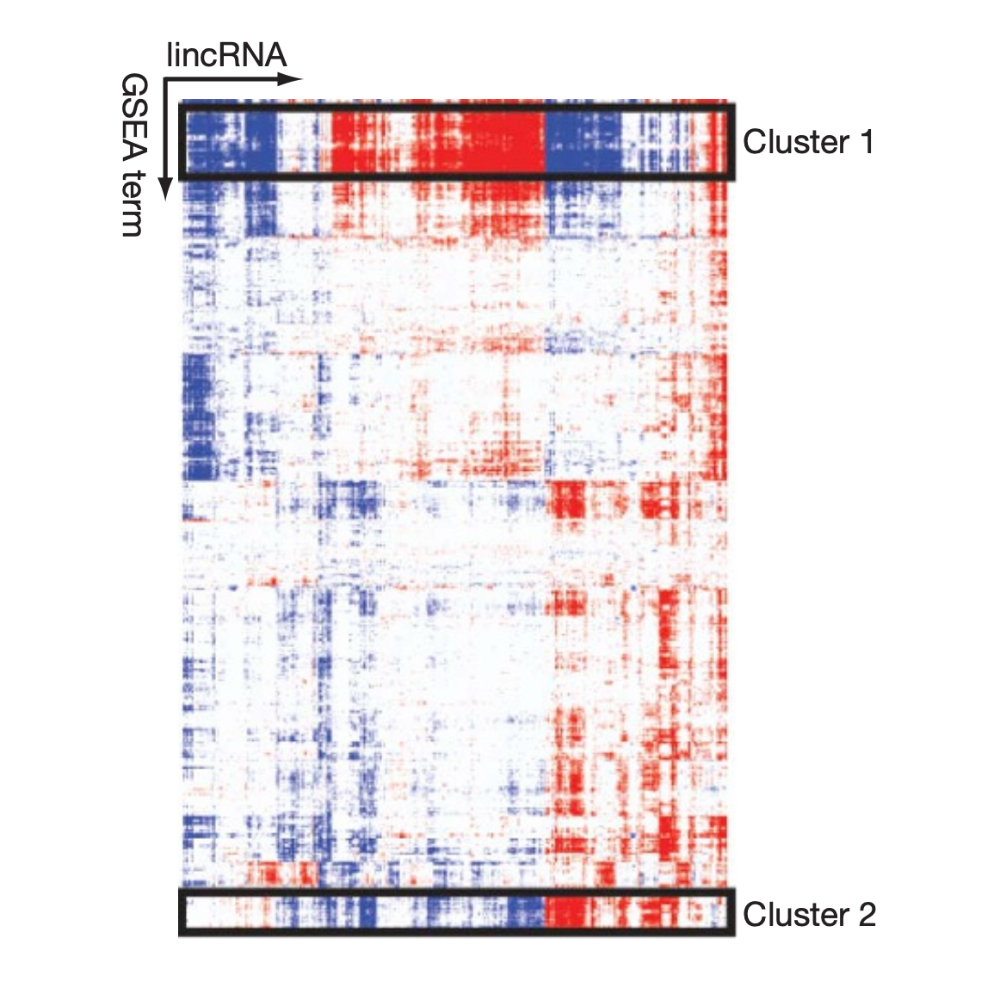
275. Population-scale tissue transcriptomics maps long non-coding RNAs to complex disease.
de, Nachun, Ferraro, Gloudemans, Rao, Smail, Eulalio, Aguet, Ng, Xu, Barbeira, Castel, Kim-Hellmuth, Park, Scott, Strober, Brown, Wen, Hall, Battle, Lappalainen, Im, Ardlie, Mostafavi, Quertermous, Kirkegaard, Montgomery
Long non-coding RNA (lncRNA) genes have well-established and important impacts on molecular and cellular functions. However, among the thousands of lncRNA genes, it is still a major challenge to identify the subset with disease or trait relevance. To systematically characterize these lncRNA genes, we used Genotype Tissue Expression (GTEx) project v8 genetic and multi-tissue transcriptomic data to profile the expression, genetic regulation, cellular contexts, and trait associations of 14,100 lncRNA genes across 49 tissues for 101 distinct complex genetic traits. Using these approaches, we identified 1,432 lncRNA gene-trait associations, 800 of which were not explained by stronger effects of neighboring protein-coding genes. This included associations between lncRNA quantitative trait loci and inflammatory bowel disease, type 1 and type 2 diabetes, and coronary artery disease, as well as rare variant associations to body mass index.
Journal: Cell, 2021 May 13 DOI: 10.1016/j.cell.2021.03.050 PMID: 33864768.0 PMCID: PMC8651477
274. SARS-CoV-2 gene content and COVID-19 mutation impact by comparing 44 Sarbecovirus genomes.
Jungreis, Sealfon, Kellis
Despite its clinical importance, the SARS-CoV-2 gene set remains unresolved, hindering dissection of COVID-19 biology. We use comparative genomics to provide a high-confidence protein-coding gene set, characterize evolutionary constraint, and prioritize functional mutations. We select 44 Sarbecovirus genomes at ideally-suited evolutionary distances, and quantify protein-coding evolutionary signatures and overlapping constraint. We find strong protein-coding signatures for ORFs 3a, 6, 7a, 7b, 8, 9b, and a novel alternate-frame gene, ORF3c, whereas ORFs 2b, 3d/3d-2, 3b, 9c, and 10 lack protein-coding signatures or convincing experimental evidence of protein-coding function. Furthermore, we show no other conserved protein-coding genes remain to be discovered. Mutation analysis suggests ORF8 contributes to within-individual fitness but not person-to-person transmission. Cross-strain and within-strain evolutionary pressures agree, except for fewer-than-expected within-strain mutations in nsp3 and S1, and more-than-expected in nucleocapsid, which shows a cluster of mutations in a predicted B-cell epitope, suggesting immune-avoidance selection. Evolutionary histories of residues disrupted by spike-protein substitutions D614G, N501Y, E484K, and K417N/T provide clues about their biology, and we catalog likely-functional co-inherited mutations. Previously reported RNA-modification sites show no enrichment for conservation. Here we report a high-confidence gene set and evolutionary-history annotations providing valuable resources and insights on SARS-CoV-2 biology, mutations, and evolution.
Journal: Nature Communications, 2021 May 11 DOI: 10.1038/s41467-021-22905-7 PMID: 33976134.0 PMCID: PMC8113528
273. Model-based assessment of replicability for genome-wide association meta-analysis.
McGuire, Jiang, Liu, Weissenkampen, Eckert, Yang, Chen, Berg, Vrieze, Jiang, Li, Liu
Genome-wide association meta-analysis (GWAMA) is an effective approach to enlarge sample sizes and empower the discovery of novel associations between genotype and phenotype. Independent replication has been used as a gold-standard for validating genetic associations. However, as current GWAMA often seeks to aggregate all available datasets, it becomes impossible to find a large enough independent dataset to replicate new discoveries. Here we introduce a method, MAMBA (Meta-Analysis Model-based Assessment of replicability), for assessing the "posterior-probability-of-replicability" for identified associations by leveraging the strength and consistency of association signals between contributing studies. We demonstrate using simulations that MAMBA is more powerful and robust than existing methods, and produces more accurate genetic effects estimates. We apply MAMBA to a large-scale meta-analysis of addiction phenotypes with 1.2 million individuals. In addition to accurately identifying replicable common variant associations, MAMBA also pinpoints novel replicable rare variant associations from imputation-based GWAMA and hence greatly expands the set of analyzable variants.
Journal: Nature Communications, 2021 Mar 30 DOI: 10.1038/s41467-021-21226-z PMID: 33785739.0 PMCID: PMC8009871
272. APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia.
Sienski, Narayan, Bonner, Kory, Boland, Arczewska, Ralvenius, Akay, Lockshin, He, Milo, Graziosi, Baru, Lewis, Kellis, Sabatini, Tsai, Lindquist
The E4 allele of the apolipoprotein E gene (APOE) has been established as a genetic risk factor for many diseases including cardiovascular diseases and Alzheimer's disease (AD), yet its mechanism of action remains poorly understood. APOE is a lipid transport protein, and the dysregulation of lipids has recently emerged as a key feature of several neurodegenerative diseases including AD. However, it is unclear how APOE4 perturbs the intracellular lipid state. Here, we report that APOE4, but not APOE3, disrupted the cellular lipidomes of human induced pluripotent stem cell (iPSC)-derived astrocytes generated from fibroblasts of APOE4 or APOE3 carriers, and of yeast expressing human APOE isoforms. We combined lipidomics and unbiased genome-wide screens in yeast with functional and genetic characterization to demonstrate that human APOE4 induced altered lipid homeostasis. These changes resulted in increased unsaturation of fatty acids and accumulation of intracellular lipid droplets both in yeast and in APOE4-expressing human iPSC-derived astrocytes. We then identified genetic and chemical modulators of this lipid disruption. We showed that supplementation of the culture medium with choline (a soluble phospholipid precursor) restored the cellular lipidome to its basal state in APOE4-expressing human iPSC-derived astrocytes and in yeast expressing human APOE4 Our study illuminates key molecular disruptions in lipid metabolism that may contribute to the disease risk linked to the APOE4 genotype. Our study suggests that manipulating lipid metabolism could be a therapeutic approach to help alleviate the consequences of carrying the APOE4 allele.
Journal: Science Translational Medicine, 2021 Mar 3 DOI: 10.1126/scitranslmed.aaz4564 PMID: 33658354.0 PMCID: PMC8218593
271. Exome-wide age-of-onset analysis reveals exonic variants in ERN1 and SPPL2C associated with Alzheimer's disease.
He, Loika, Park, Bennett, Kellis, Kulminski
Despite recent discoveries in genome-wide association studies (GWAS) of genomic variants associated with Alzheimer's disease (AD), its underlying biological mechanisms are still elusive. The discovery of novel AD-associated genetic variants, particularly in coding regions and from APOE epsilon4 non-carriers, is critical for understanding the pathology of AD. In this study, we carried out an exome-wide association analysis of age-of-onset of AD with ~20,000 subjects and placed more emphasis on APOE epsilon4 non-carriers. Using Cox mixed-effects models, we find that age-of-onset shows a stronger genetic signal than AD case-control status, capturing many known variants with stronger significance, and also revealing new variants. We identified two novel variants, rs56201815, a rare synonymous variant in ERN1, and rs12373123, a common missense variant in SPPL2C in the MAPT region in APOE epsilon4 non-carriers. Besides, a rare missense variant rs144292455 in TACR3 showed the consistent direction of effect sizes across all studies with a suggestive significant level. In an attempt to unravel their regulatory and biological functions, we found that the minor allele of rs56201815 was associated with lower average FDG uptake across five brain regions in ADNI. Our eQTL analyses based on 6198 gene expression samples from ROSMAP and GTEx revealed that the minor allele of rs56201815 was potentially associated with elevated expression of ERN1, a key gene triggering unfolded protein response (UPR), in multiple brain regions, including the posterior cingulate cortex and nucleus accumbens. Our cell-type-specific eQTL analysis using ~80,000 single nuclei in the prefrontal cortex revealed that the protective minor allele of rs12373123 significantly increased the expression of GRN in microglia, and was associated with MAPT expression in astrocytes. These findings provide novel evidence supporting the hypothesis of the potential involvement of the UPR to ER stress in the pathological pathway of AD, and also give more insights into underlying regulatory mechanisms behind the pleiotropic effects of rs12373123 in multiple degenerative diseases including AD and Parkinson's disease.
Journal: Translational Psychiatry, 2021 Feb 26 DOI: 10.1038/s41398-021-01263-4 PMID: 33637690.0 PMCID: PMC7910483
270. Distinct metabolic programs established in the thymus control effector functions of gammadelta T cell subsets in tumor microenvironments.
Lopes, McIntyre, Martin, Raverdeau, Sumaria, Kohlgruber, Fiala, Agudelo, Dyck, Kane, Douglas, Cunningham, Prendeville, Loftus, Carmody, Pierre, Kellis, Brenner, Arguello, Silva-Santos, Pennington, Lynch
Metabolic programming controls immune cell lineages and functions, but little is known about gammadelta T cell metabolism. Here, we found that gammadelta T cell subsets making either interferon-gamma (IFN-gamma) or interleukin (IL)-17 have intrinsically distinct metabolic requirements. Whereas IFN-gamma(+) gammadelta T cells were almost exclusively dependent on glycolysis, IL-17(+) gammadelta T cells strongly engaged oxidative metabolism, with increased mitochondrial mass and activity. These distinct metabolic signatures were surprisingly imprinted early during thymic development and were stably maintained in the periphery and within tumors. Moreover, pro-tumoral IL-17(+) gammadelta T cells selectively showed high lipid uptake and intracellular lipid storage and were expanded in obesity and in tumors of obese mice. Conversely, glucose supplementation enhanced the antitumor functions of IFN-gamma(+) gammadelta T cells and reduced tumor growth upon adoptive transfer. These findings have important implications for the differentiation of effector gammadelta T cells and their manipulation in cancer immunotherapy.
Journal: Nature Immunology, 2021 Feb DOI: 10.1038/s41590-020-00848-3 PMID: 33462452.0 PMCID: PMC7610600
269. A wealth of discovery built on the Human Genome Project - by the numbers.
Gates, Gysi, Kellis, Barabasi
Journal: Nature, 2021 Feb DOI: 10.1038/d41586-021-00314-6 PMID: 33568828.0
268. Regulatory genomic circuitry of human disease loci by integrative epigenomics.
Boix, James, Park, Meuleman, Kellis
Annotating the molecular basis of human disease remains an unsolved challenge, as 93% of disease loci are non-coding and gene-regulatory annotations are highly incomplete(1-3). Here we present EpiMap, a compendium comprising 10,000 epigenomic maps across 800 samples, which we used to define chromatin states, high-resolution enhancers, enhancer modules, upstream regulators and downstream target genes. We used this resource to annotate 30,000 genetic loci that were associated with 540 traits(4), predicting trait-relevant tissues, putative causal nucleotide variants in enriched tissue enhancers and candidate tissue-specific target genes for each. We partitioned multifactorial traits into tissue-specific contributing factors with distinct functional enrichments and disease comorbidity patterns, and revealed both single-factor monotropic and multifactor pleiotropic loci. Top-scoring loci frequently had multiple predicted driver variants, converging through multiple enhancers with a common target gene, multiple genes in common tissues, or multiple genes and multiple tissues, indicating extensive pleiotropy. Our results demonstrate the importance of dense, rich, high-resolution epigenomic annotations for the investigation of complex traits.
Journal: Nature, 2021 Feb DOI: 10.1038/s41586-020-03145-z PMID: 33536621.0 PMCID: PMC7875769
267. Genus-Wide Characterization of Bumblebee Genomes Provides Insights into Their Evolution and Variation in Ecological and Behavioral Traits.
Sun, Huang, Wang, Zhao, Su, Thomas, Zhao, Zhang, Jungreis, Kellis, Vicario, Sharakhov, Bondarenko, Hasselmann, Kim, Paten, Penso-Dolfin, Wang, Chang, Gao, Ma, Ma, Zhang, Zhang, Zhang, Ruzzante, Robertson, Zhu, Liu, Yang, Ding, Wang, Ma, Xu, Liang, Itgen, Mee, Cao, Zhang, Sadd, Hahn, Schaack, Barribeau, Williams, Waterhouse, Mueller
Bumblebees are a diverse group of globally important pollinators in natural ecosystems and for agricultural food production. With both eusocial and solitary life-cycle phases, and some social parasite species, they are especially interesting models to understand social evolution, behavior, and ecology. Reports of many species in decline point to pathogen transmission, habitat loss, pesticide usage, and global climate change, as interconnected causes. These threats to bumblebee diversity make our reliance on a handful of well-studied species for agricultural pollination particularly precarious. To broadly sample bumblebee genomic and phenotypic diversity, we de novo sequenced and assembled the genomes of 17 species, representing all 15 subgenera, producing the first genus-wide quantification of genetic and genomic variation potentially underlying key ecological and behavioral traits. The species phylogeny resolves subgenera relationships, whereas incomplete lineage sorting likely drives high levels of gene tree discordance. Five chromosome-level assemblies show a stable 18-chromosome karyotype, with major rearrangements creating 25 chromosomes in social parasites. Differential transposable element activity drives changes in genome sizes, with putative domestications of repetitive sequences influencing gene coding and regulatory potential. Dynamically evolving gene families and signatures of positive selection point to genus-wide variation in processes linked to foraging, diet and metabolism, immunity and detoxification, as well as adaptations for life at high altitudes. Our study reveals how bumblebee genes and genomes have evolved across the Bombus phylogeny and identifies variations potentially linked to key ecological and behavioral traits of these important pollinators.
Journal: Molecular Biology And Evolution, 2021 Jan 23 DOI: 10.1093/molbev/msaa240 PMID: 32946576.0 PMCID: PMC7826183
266. GENCODE 2021.
Frankish, Diekhans, Jungreis, Lagarde, Loveland, Mudge, Sisu, Wright, Armstrong, Barnes, Berry, Bignell, Boix, Carbonell, Cunningham, Di, Donaldson, Fiddes, Garcia, Gonzalez, Grego, Hardy, Hourlier, Howe, Hunt, Izuogu, Johnson, Martin, Martinez, Mohanan, Muir, Navarro, Parker, Pei, Pozo, Riera, Ruffier, Schmitt, Stapleton, Suner, Sycheva, Uszczynska-Ratajczak, Wolf, Xu, Yang, Yates, Zerbino, Zhang, Choudhary, Gerstein, Guigo, Hubbard, Kellis, Paten, Tress, Flicek
The GENCODE project annotates human and mouse genes and transcripts supported by experimental data with high accuracy, providing a foundational resource that supports genome biology and clinical genomics. GENCODE annotation processes make use of primary data and bioinformatic tools and analysis generated both within the consortium and externally to support the creation of transcript structures and the determination of their function. Here, we present improvements to our annotation infrastructure, bioinformatics tools, and analysis, and the advances they support in the annotation of the human and mouse genomes including: the completion of first pass manual annotation for the mouse reference genome; targeted improvements to the annotation of genes associated with SARS-CoV-2 infection; collaborative projects to achieve convergence across reference annotation databases for the annotation of human and mouse protein-coding genes; and the first GENCODE manually supervised automated annotation of lncRNAs. Our annotation is accessible via Ensembl, the UCSC Genome Browser and https://www.gencodegenes.org.
Journal: Nucleic Acids Research, 2021 Jan 8 DOI: 10.1093/nar/gkaa1087 PMID: 33270111.0 PMCID: PMC7778937
265. Genomic RNA Elements Drive Phase Separation of the SARS-CoV-2 Nucleocapsid.
Iserman, Roden, Boerneke, Sealfon, McLaughlin, Jungreis, Fritch, Hou, Ekena, Weidmann, Theesfeld, Kellis, Troyanskaya, Baric, Sheahan, Weeks, Gladfelter
We report that the SARS-CoV-2 nucleocapsid protein (N-protein) undergoes liquid-liquid phase separation (LLPS) with viral RNA. N-protein condenses with specific RNA genomic elements under physiological buffer conditions and condensation is enhanced at human body temperatures (33 degrees C and 37 degrees C) and reduced at room temperature (22 degrees C). RNA sequence and structure in specific genomic regions regulate N-protein condensation while other genomic regions promote condensate dissolution, potentially preventing aggregation of the large genome. At low concentrations, N-protein preferentially crosslinks to specific regions characterized by single-stranded RNA flanked by structured elements and these features specify the location, number, and strength of N-protein binding sites (valency). Liquid-like N-protein condensates form in mammalian cells in a concentration-dependent manner and can be altered by small molecules. Condensation of N-protein is RNA sequence and structure specific, sensitive to human body temperature, and manipulatable with small molecules, and therefore presents a screenable process for identifying antiviral compounds effective against SARS-CoV-2.
Journal: Molecular Cell, 2020 Dec 17 DOI: 10.1016/j.molcel.2020.11.041 PMID: 33290746.0 PMCID: PMC7691212
264. Conserved Epigenetic Regulatory Logic Infers Genes Governing Cell Identity.
Shim, Sinniah, Xu, Vitrinel, Alexanian, Andreoletti, Shen, Sun, Balderson, Boix, Peng, Jing, Wang, Kellis, Tam, Smith, Piper, Christiaen, Nguyen, Boden, Palpant
Determining genes that orchestrate cell differentiation in development and disease remains a fundamental goal of cell biology. This study establishes a genome-wide metric based on the gene-repressive trimethylation of histone H3 at lysine 27 (H3K27me3) across hundreds of diverse cell types to identify genetic regulators of cell differentiation. We introduce a computational method, TRIAGE, which uses discordance between gene-repressive tendency and expression to identify genetic drivers of cell identity. We apply TRIAGE to millions of genome-wide single-cell transcriptomes, diverse omics platforms, and eukaryotic cells and tissue types. Using a wide range of data, we validate the performance of TRIAGE in identifying cell-type-specific regulatory factors across diverse species including human, mouse, boar, bird, fish, and tunicate. Using CRISPR gene editing, we use TRIAGE to experimentally validate RNF220 as a regulator of Ciona cardiopharyngeal development and SIX3 as required for differentiation of endoderm in human pluripotent stem cells. A record of this paper's transparent peer review process is included in the Supplemental Information.
Journal: Cell Systems, 2020 Dec 16 DOI: 10.1016/j.cels.2020.11.001 PMID: 33278344.0 PMCID: PMC7781436
263. Mapping the epigenomic and transcriptomic interplay during memory formation and recall in the hippocampal engram ensemble.
Marco, Meharena, Dileep, Raju, Davila-Velderrain, Zhang, Adaikkan, Young, Gao, Kellis, Tsai
The epigenome and three-dimensional (3D) genomic architecture are emerging as key factors in the dynamic regulation of different transcriptional programs required for neuronal functions. In this study, we used an activity-dependent tagging system in mice to determine the epigenetic state, 3D genome architecture and transcriptional landscape of engram cells over the lifespan of memory formation and recall. Our findings reveal that memory encoding leads to an epigenetic priming event, marked by increased accessibility of enhancers without the corresponding transcriptional changes. Memory consolidation subsequently results in spatial reorganization of large chromatin segments and promoter-enhancer interactions. Finally, with reactivation, engram neurons use a subset of de novo long-range interactions, where primed enhancers are brought in contact with their respective promoters to upregulate genes involved in local protein translation in synaptic compartments. Collectively, our work elucidates the comprehensive transcriptional and epigenomic landscape across the lifespan of memory formation and recall in the hippocampal engram ensemble.
Journal: Nature Neuroscience, 2020 Dec DOI: 10.1038/s41593-020-00717-0 PMID: 33020654.0 PMCID: PMC7686266
262. Plasma-derived extracellular vesicle analysis and deconvolution enable prediction and tracking of melanoma checkpoint blockade outcome.
Shi, Kasumova, Michaud, Cintolo-Gonzalez, Diaz-Martinez, Ohmura, Mehta, Chien, Frederick, Cohen, Plana, Johnson, Flaherty, Sullivan, Kellis, Boland
Immune checkpoint inhibitors (ICIs) show promise, but most patients do not respond. We identify and validate biomarkers from extracellular vesicles (EVs), allowing non-invasive monitoring of tumor- intrinsic and host immune status, as well as a prediction of ICI response. We undertook transcriptomic profiling of plasma-derived EVs and tumors from 50 patients with metastatic melanoma receiving ICI, and validated with an independent EV-only cohort of 30 patients. Plasma-derived EV and tumor transcriptomes correlate. EV profiles reveal drivers of ICI resistance and melanoma progression, exhibit differentially expressed genes/pathways, and correlate with clinical response to ICI. We created a Bayesian probabilistic deconvolution model to estimate contributions from tumor and non-tumor sources, enabling interpretation of differentially expressed genes/pathways. EV RNA-seq mutations also segregated ICI response. EVs serve as a non-invasive biomarker to jointly probe tumor-intrinsic and immune changes to ICI, function as predictive markers of ICI responsiveness, and monitor tumor persistence and immune activation.
Journal: Science Advances, 2020 Nov DOI: 10.1126/sciadv.abb3461 PMID: 33188016.0 PMCID: PMC7673759
261. Evidence for secondary-variant genetic burden and non-random distribution across biological modules in a recessive ciliopathy.
Kousi, Soylemez, Ozanturk, Mourtzi, Akle, Jungreis, Muller, Cassa, Brand, Mokry, Wolf, Sadeghpour, McFadden, Lewis, Talkowski, Dollfus, Kellis, Davis, Sunyaev, Katsanis
The influence of genetic background on driver mutations is well established; however, the mechanisms by which the background interacts with Mendelian loci remain unclear. We performed a systematic secondary-variant burden analysis of two independent cohorts of patients with Bardet-Biedl syndrome (BBS) with known recessive biallelic pathogenic mutations in one of 17 BBS genes for each individual. We observed a significant enrichment of trans-acting rare nonsynonymous secondary variants in patients with BBS compared with either population controls or a cohort of individuals with a non-BBS diagnosis and recessive variants in the same gene set. Strikingly, we found a significant over-representation of secondary alleles in chaperonin-encoding genes-a finding corroborated by the observation of epistatic interactions involving this complex in vivo. These data indicate a complex genetic architecture for BBS that informs the biological properties of disease modules and presents a model for secondary-variant burden analysis in recessive disorders.
Journal: Nature Genetics, 2020 Nov DOI: 10.1038/s41588-020-0707-1 PMID: 33046855.0 PMCID: PMC8272915
260. A multiresolution framework to characterize single-cell state landscapes.
Mohammadi, Davila-Velderrain, Kellis
Dissecting the cellular heterogeneity embedded in single-cell transcriptomic data is challenging. Although many methods and approaches exist, identifying cell states and their underlying topology is still a major challenge. Here, we introduce the concept of multiresolution cell-state decomposition as a practical approach to simultaneously capture both fine- and coarse-grain patterns of variability. We implement this concept in ACTIONet, a comprehensive framework that combines archetypal analysis and manifold learning to provide a ready-to-use analytical approach for multiresolution single-cell state characterization. ACTIONet provides a robust, reproducible, and highly interpretable single-cell analysis platform that couples dominant pattern discovery with a corresponding structural representation of the cell state landscape. Using multiple synthetic and real data sets, we demonstrate ACTIONet's superior performance relative to existing alternatives. We use ACTIONet to integrate and annotate cells across three human cortex data sets. Through integrative comparative analysis, we define a consensus vocabulary and a consistent set of gene signatures discriminating against the transcriptomic cell types and subtypes of the human prefrontal cortex.
Journal: Nature Communications, 2020 Oct 26 DOI: 10.1038/s41467-020-18416-6 PMID: 33106496.0 PMCID: PMC7588427
259. SARS-CoV-2 gene content and COVID-19 mutation impact by comparing 44 Sarbecovirus genomes.
Jungreis, Sealfon, Kellis
Despite its overwhelming clinical importance, the SARS-CoV-2 gene set remains unresolved, hindering dissection of COVID-19 biology. Here, we use comparative genomics to provide a high-confidence protein-coding gene set, characterize protein-level and nucleotide-level evolutionary constraint, and prioritize functional mutations from the ongoing COVID-19 pandemic. We select 44 complete Sarbecovirus genomes at evolutionary distances ideally-suited for protein-coding and non-coding element identification, create whole-genome alignments, and quantify protein-coding evolutionary signatures and overlapping constraint. We find strong protein-coding signatures for all named genes and for 3a, 6, 7a, 7b, 8, 9b, and also ORF3c, a novel alternate-frame gene. By contrast, ORF10, and overlapping-ORFs 9c, 3b, and 3d lack protein-coding signatures or convincing experimental evidence and are not protein-coding. Furthermore, we show no other protein-coding genes remain to be discovered. Cross-strain and within-strain evolutionary pressures largely agree at the gene, amino-acid, and nucleotide levels, with some notable exceptions, including fewer-than-expected mutations in nsp3 and Spike subunit S1, and more-than-expected mutations in Nucleocapsid. The latter also shows a cluster of amino-acid-changing variants in otherwise-conserved residues in a predicted B-cell epitope, which may indicate positive selection for immune avoidance. Several Spike-protein mutations, including D614G, which has been associated with increased transmission, disrupt otherwise-perfectly-conserved amino acids, and could be novel adaptations to human hosts. The resulting high-confidence gene set and evolutionary-history annotations provide valuable resources and insights on COVID-19 biology, mutations, and evolution.
Journal: Research Square, 2020 Oct 1 DOI: 10.21203/rs.3.rs-80345/v1 PMID: 33024961.0 PMCID: PMC7536840
258. A Quantitative Proteome Map of the Human Body.
Jiang, Wang, Lin, Jian, Li, Chan, Dong, Fang, Robinson, Snyder
Determining protein levels in each tissue and how they compare with RNA levels is important for understanding human biology and disease as well as regulatory processes that control protein levels. We quantified the relative protein levels from over 12,000 genes across 32 normal human tissues. Tissue-specific or tissue-enriched proteins were identified and compared to transcriptome data. Many ubiquitous transcripts are found to encode tissue-specific proteins. Discordance of RNA and protein enrichment revealed potential sites of synthesis and action of secreted proteins. The tissue-specific distribution of proteins also provides an in-depth view of complex biological events that require the interplay of multiple tissues. Most importantly, our study demonstrated that protein tissue-enrichment information can explain phenotypes of genetic diseases, which cannot be obtained by transcript information alone. Overall, our results demonstrate how understanding protein levels can provide insights into regulation, secretome, metabolism, and human diseases.
Journal: Cell, 2020 Oct 1 DOI: 10.1016/j.cell.2020.08.036 PMID: 32916130.0 PMCID: PMC7575058
257. Retrospective evaluation of whole exome and genome mutation calls in 746 cancer samples.
Bailey, Meyerson, Dursi, Wang, Dong, Liang, Weerasinghe, Li, Li, Kelso, Saksena, Ellrott, Wendl, Wheeler, Getz, Simpson, Gerstein, Ding
The Cancer Genome Atlas (TCGA) and International Cancer Genome Consortium (ICGC) curated consensus somatic mutation calls using whole exome sequencing (WES) and whole genome sequencing (WGS), respectively. Here, as part of the ICGC/TCGA Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium, which aggregated whole genome sequencing data from 2,658 cancers across 38 tumour types, we compare WES and WGS side-by-side from 746 TCGA samples, finding that ~80% of mutations overlap in covered exonic regions. We estimate that low variant allele fraction (VAF < 15%) and clonal heterogeneity contribute up to 68% of private WGS mutations and 71% of private WES mutations. We observe that ~30% of private WGS mutations trace to mutations identified by a single variant caller in WES consensus efforts. WGS captures both ~50% more variation in exonic regions and un-observed mutations in loci with variable GC-content. Together, our analysis highlights technological divergences between two reproducible somatic variant detection efforts.
Journal: Nature Communications, 2020 Sep 21 DOI: 10.1038/s41467-020-18151-y PMID: 32958763.0 PMCID: PMC7505971
256. Improved haplotype inference by exploiting long-range linking and allelic imbalance in RNA-seq datasets.
Berger, Yorukoglu, Zhang, Nyquist, Shalek, Kellis, Numanagic, Berger
Haplotype reconstruction of distant genetic variants remains an unsolved problem due to the short-read length of common sequencing data. Here, we introduce HapTree-X, a probabilistic framework that utilizes latent long-range information to reconstruct unspecified haplotypes in diploid and polyploid organisms. It introduces the observation that differential allele-specific expression can link genetic variants from the same physical chromosome, thus even enabling using reads that cover only individual variants. We demonstrate HapTree-X's feasibility on in-house sequenced Genome in a Bottle RNA-seq and various whole exome, genome, and 10X Genomics datasets. HapTree-X produces more complete phases (up to 25%), even in clinically important genes, and phases more variants than other methods while maintaining similar or higher accuracy and being up to 10x faster than other tools. The advantage of HapTree-X's ability to use multiple lines of evidence, as well as to phase polyploid genomes in a single integrative framework, substantially grows as the amount of diverse data increases.
Journal: Nature Communications, 2020 Sep 16 DOI: 10.1038/s41467-020-18320-z PMID: 32938926.0 PMCID: PMC7494856
255. The GTEx Consortium atlas of genetic regulatory effects across human tissues.
The GTEx Consortium
The Genotype-Tissue Expression (GTEx) project was established to characterize genetic effects on the transcriptome across human tissues and to link these regulatory mechanisms to trait and disease associations. Here, we present analyses of the version 8 data, examining 15,201 RNA-sequencing samples from 49 tissues of 838 postmortem donors. We comprehensively characterize genetic associations for gene expression and splicing in cis and trans, showing that regulatory associations are found for almost all genes, and describe the underlying molecular mechanisms and their contribution to allelic heterogeneity and pleiotropy of complex traits. Leveraging the large diversity of tissues, we provide insights into the tissue specificity of genetic effects and show that cell type composition is a key factor in understanding gene regulatory mechanisms in human tissues.
Journal: Science, 2020 Sep 11 DOI: 10.1126/science.aaz1776 PMID: 32913098.0 PMCID: PMC7737656
254. Cell type-specific genetic regulation of gene expression across human tissues.
Kim-Hellmuth, Aguet, Oliva, Munoz-Aguirre, Kasela, Wucher, Castel, Hamel, Vinuela, Roberts, Mangul, Wen, Wang, Barbeira, Garrido-Martin, Nadel, Zou, Bonazzola, Quan, Brown, Martinez-Perez, Soria, Getz, Dermitzakis, Small, Stephens, Xi, Im, Guigo, Segre, Stranger, Ardlie, Lappalainen
The Genotype-Tissue Expression (GTEx) project has identified expression and splicing quantitative trait loci in cis (QTLs) for the majority of genes across a wide range of human tissues. However, the functional characterization of these QTLs has been limited by the heterogeneous cellular composition of GTEx tissue samples. We mapped interactions between computational estimates of cell type abundance and genotype to identify cell type-interaction QTLs for seven cell types and show that cell type-interaction expression QTLs (eQTLs) provide finer resolution to tissue specificity than bulk tissue cis-eQTLs. Analyses of genetic associations with 87 complex traits show a contribution from cell type-interaction QTLs and enables the discovery of hundreds of previously unidentified colocalized loci that are masked in bulk tissue.
Journal: Science, 2020 Sep 11 DOI: 10.1126/science.aaz8528 PMID: 32913075.0 PMCID: PMC8051643
253. Determinants of telomere length across human tissues.
Demanelis, Jasmine, Chen, Chernoff, Tong, Delgado, Zhang, Shinkle, Sabarinathan, Lin, Ramirez, Oliva, Kim-Hellmuth, Stranger, Lai, Aviv, Ardlie, Aguet, Ahsan, Doherty, Kibriya, Pierce
Telomere shortening is a hallmark of aging. Telomere length (TL) in blood cells has been studied extensively as a biomarker of human aging and disease; however, little is known regarding variability in TL in nonblood, disease-relevant tissue types. Here, we characterize variability in TLs from 6391 tissue samples, representing >20 tissue types and 952 individuals from the Genotype-Tissue Expression (GTEx) project. We describe differences across tissue types, positive correlation among tissue types, and associations with age and ancestry. We show that genetic variation affects TL in multiple tissue types and that TL may mediate the effect of age on gene expression. Our results provide the foundational knowledge regarding TL in healthy tissues that is needed to interpret epidemiological studies of TL and human health.
Journal: Science, 2020 Sep 11 DOI: 10.1126/science.aaz6876 PMID: 32913074.0 PMCID: PMC8108546
252. Transcriptomic signatures across human tissues identify functional rare genetic variation.
Ferraro, Strober, Einson, Abell, Aguet, Barbeira, Brandt, Bucan, Castel, Davis, Greenwald, Hess, Hilliard, Kember, Kotis, Park, Peloso, Ramdas, Scott, Smail, Tsang, Zekavat, Ziosi, Aradhana, Ardlie, Assimes, Bassik, Brown, Correa, Hall, Im, Li, Natarajan, Lappalainen, Mohammadi, Montgomery, Battle
Rare genetic variants are abundant across the human genome, and identifying their function and phenotypic impact is a major challenge. Measuring aberrant gene expression has aided in identifying functional, large-effect rare variants (RVs). Here, we expanded detection of genetically driven transcriptome abnormalities by analyzing gene expression, allele-specific expression, and alternative splicing from multitissue RNA-sequencing data, and demonstrate that each signal informs unique classes of RVs. We developed Watershed, a probabilistic model that integrates multiple genomic and transcriptomic signals to predict variant function, validated these predictions in additional cohorts and through experimental assays, and used them to assess RVs in the UK Biobank, the Million Veterans Program, and the Jackson Heart Study. Our results link thousands of RVs to diverse molecular effects and provide evidence to associate RVs affecting the transcriptome with human traits.
Journal: Science, 2020 Sep 11 DOI: 10.1126/science.aaz5900 PMID: 32913073.0 PMCID: PMC7646251
251. The impact of sex on gene expression across human tissues.
Oliva, Munoz-Aguirre, Kim-Hellmuth, Wucher, Gewirtz, Cotter, Parsana, Kasela, Balliu, Vinuela, Castel, Mohammadi, Aguet, Zou, Khramtsova, Skol, Garrido-Martin, Reverter, Brown, Evans, Gamazon, Payne, Bonazzola, Barbeira, Hamel, Martinez-Perez, Soria, Pierce, Stephens, Eskin, Dermitzakis, Segre, Im, Engelhardt, Ardlie, Montgomery, Battle, Lappalainen, Guigo, Stranger
Many complex human phenotypes exhibit sex-differentiated characteristics. However, the molecular mechanisms underlying these differences remain largely unknown. We generated a catalog of sex differences in gene expression and in the genetic regulation of gene expression across 44 human tissue sources surveyed by the Genotype-Tissue Expression project (GTEx, v8 release). We demonstrate that sex influences gene expression levels and cellular composition of tissue samples across the human body. A total of 37% of all genes exhibit sex-biased expression in at least one tissue. We identify cis expression quantitative trait loci (eQTLs) with sex-differentiated effects and characterize their cellular origin. By integrating sex-biased eQTLs with genome-wide association study data, we identify 58 gene-trait associations that are driven by genetic regulation of gene expression in a single sex. These findings provide an extensive characterization of sex differences in the human transcriptome and its genetic regulation.
Journal: Science, 2020 Sep 11 DOI: 10.1126/science.aba3066 PMID: 32913072.0 PMCID: PMC8136152
250. A vast resource of allelic expression data spanning human tissues.
Castel, Aguet, Mohammadi, Ardlie, Lappalainen
Allele expression (AE) analysis robustly measures cis-regulatory effects. Here, we present and demonstrate the utility of a vast AE resource generated from the GTEx v8 release, containing 15,253 samples spanning 54 human tissues for a total of 431 million measurements of AE at the SNP level and 153 million measurements at the haplotype level. In addition, we develop an extension of our tool phASER that allows effect sizes of cis-regulatory variants to be estimated using haplotype-level AE data. This AE resource is the largest to date, and we are able to make haplotype-level data publicly available. We anticipate that the availability of this resource will enable future studies of regulatory variation across human tissues.
Journal: Genome Biology, 2020 Sep 11 DOI: 10.1186/s13059-020-02122-z PMID: 32912332.0 PMCID: PMC7488534
249. Cell Type-Specific Transcriptomics Reveals that Mutant Huntingtin Leads to Mitochondrial RNA Release and Neuronal Innate Immune Activation.
Lee, Fenster, Pineda, Gibbs, Mohammadi, Davila-Velderrain, Garcia, Therrien, Novis, Gao, Wilkinson, Vogt, Kellis, LaVoie, Heiman
The mechanisms by which mutant huntingtin (mHTT) leads to neuronal cell death in Huntington's disease (HD) are not fully understood. To gain new molecular insights, we used single nuclear RNA sequencing (snRNA-seq) and translating ribosome affinity purification (TRAP) to conduct transcriptomic analyses of caudate/putamen (striatal) cell type-specific gene expression changes in human HD and mouse models of HD. In striatal spiny projection neurons, the most vulnerable cell type in HD, we observe a release of mitochondrial RNA (mtRNA) (a potent mitochondrial-derived innate immunogen) and a concomitant upregulation of innate immune signaling in spiny projection neurons. Further, we observe that the released mtRNAs can directly bind to the innate immune sensor protein kinase R (PKR). We highlight the importance of studying cell type-specific gene expression dysregulation in HD pathogenesis and reveal that the activation of innate immune signaling in the most vulnerable HD neurons provides a novel framework to understand the basis of mHTT toxicity and raises new therapeutic opportunities.
Journal: Neuron, 2020 Sep 9 DOI: 10.1016/j.neuron.2020.06.021 PMID: 32681824.0 PMCID: PMC7486278
248. Alternatives to amyloid for Alzheimer's disease therapies-a symposium report.
Cable, Holtzman, Hyman, Tansey, Colonna, Kellis, Brinton, Albert, Wellington, Sisodia, Tanzi
For decades, Alzheimer's disease research has focused on amyloid as the primary pathogenic agent. This focus has driven the development of numerous amyloid-targeting therapies; however, with one possible exception, none of these therapies have been effective in preventing or delaying cognitive decline in patients, and there are no approved disease-modifying agents. It is becoming more apparent that alternative drug targets are needed to address this complex disease. An increased understanding of Alzheimer's disease pathology has highlighted the need to target the appropriate disease pathology at the appropriate time in the disease course. Preclinical and early clinical studies have focused on targets, including inflammation, tau, vascular health, and the microbiome. This report summarizes the presentations from a New York Academy of Sciences' one-day symposium entitled "Alzheimer's Disease Therapeutics: Alternatives to Amyloid," held on November 20, 2019.
Journal: Annals Of The New York Academy Of Sciences, 2020 Sep DOI: 10.1111/nyas.14371 PMID: 32472577.0 PMCID: PMC9621603
247. Sex differences in oncogenic mutational processes.
Li, Prokopec, Sun, Yousif, Schmitz, Boutros
Sex differences have been observed in multiple facets of cancer epidemiology, treatment and biology, and in most cancers outside the sex organs. Efforts to link these clinical differences to specific molecular features have focused on somatic mutations within the coding regions of the genome. Here we report a pan-cancer analysis of sex differences in whole genomes of 1983 tumours of 28 subtypes as part of the ICGC/TCGA Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium. We both confirm the results of exome studies, and also uncover previously undescribed sex differences. These include sex-biases in coding and non-coding cancer drivers, mutation prevalence and strikingly, in mutational signatures related to underlying mutational processes. These results underline the pervasiveness of molecular sex differences and strengthen the call for increased consideration of sex in molecular cancer research.
Journal: Nature Communications, 2020 Aug 28 DOI: 10.1038/s41467-020-17359-2 PMID: 32859912.0 PMCID: PMC7455744
246. Translation Initiation Site Profiling Reveals Widespread Synthesis of Non-AUG-Initiated Protein Isoforms in Yeast.
Eisenberg, Higdon, Hollerer, Fields, Jungreis, Diamond, Kellis, Jovanovic, Brar
Genomic analyses in budding yeast have helped define the foundational principles of eukaryotic gene expression. However, in the absence of empirical methods for defining coding regions, these analyses have historically excluded specific classes of possible coding regions, such as those initiating at non-AUG start codons. Here, we applied an experimental approach to globally annotate translation initiation sites in yeast and identified 149 genes with alternative N-terminally extended protein isoforms initiating from near-cognate codons upstream of annotated AUG start codons. These isoforms are produced in concert with canonical isoforms and translated with high specificity, resulting from initiation at only a small subset of possible start codons. The non-AUG initiation driving their production is enriched during meiosis and induced by low eIF5A, which is seen in this context. These findings reveal widespread production of non-canonical protein isoforms and unexpected complexity to the rules by which even a simple eukaryotic genome is decoded.
Journal: Cell Systems, 2020 Aug 26 DOI: 10.1016/j.cels.2020.06.011 PMID: 32710835.0 PMCID: PMC7508262
245. Expanded encyclopaedias of DNA elements in the human and mouse genomes.
Moore, Purcaro, Pratt, Epstein, Shoresh, Adrian, Kawli, Davis, Dobin, Kaul, Halow, Van, Freese, Gorkin, Shen, He, Mackiewicz, Pauli-Behn, Williams, Mortazavi, Keller, Zhang, Elhajjajy, Huey, Dickel, Snetkova, Wei, Wang, Rivera-Mulia, Rozowsky, Zhang, Chhetri, Zhang, Victorsen, White, Visel, Yeo, Burge, Lecuyer, Gilbert, Dekker, Rinn, Mendenhall, Ecker, Kellis, Klein, Noble, Kundaje, Guigo, Farnham, Cherry, Myers, Ren, Graveley, Gerstein, Pennacchio, Snyder, Bernstein, Wold, Hardison, Gingeras, Stamatoyannopoulos, Weng
The human and mouse genomes contain instructions that specify RNAs and proteins and govern the timing, magnitude, and cellular context of their production. To better delineate these elements, phase III of the Encyclopedia of DNA Elements (ENCODE) Project has expanded analysis of the cell and tissue repertoires of RNA transcription, chromatin structure and modification, DNA methylation, chromatin looping, and occupancy by transcription factors and RNA-binding proteins. Here we summarize these efforts, which have produced 5,992 new experimental datasets, including systematic determinations across mouse fetal development. All data are available through the ENCODE data portal (https://www.encodeproject.org), including phase II ENCODE(1) and Roadmap Epigenomics(2) data. We have developed a registry of 926,535 human and 339,815 mouse candidate cis-regulatory elements, covering 7.9 and 3.4% of their respective genomes, by integrating selected datatypes associated with gene regulation, and constructed a web-based server (SCREEN; http://screen.encodeproject.org) to provide flexible, user-defined access to this resource. Collectively, the ENCODE data and registry provide an expansive resource for the scientific community to build a better understanding of the organization and function of the human and mouse genomes.
Journal: Nature, 2020 Jul DOI: 10.1038/s41586-020-2493-4 PMID: 32728249.0 PMCID: PMC7410828
244. Perspectives on ENCODE.
Snyder, Gingeras, Moore, Weng, Gerstein, Ren, Hardison, Stamatoyannopoulos, Graveley, Feingold, Pazin, Pagan, Gilchrist, Hitz, Cherry, Bernstein, Mendenhall, Zerbino, Frankish, Flicek, Myers
The Encylopedia of DNA Elements (ENCODE) Project launched in 2003 with the long-term goal of developing a comprehensive map of functional elements in the human genome. These included genes, biochemical regions associated with gene regulation (for example, transcription factor binding sites, open chromatin, and histone marks) and transcript isoforms. The marks serve as sites for candidate cis-regulatory elements (cCREs) that may serve functional roles in regulating gene expression(1). The project has been extended to model organisms, particularly the mouse. In the third phase of ENCODE, nearly a million and more than 300,000 cCRE annotations have been generated for human and mouse, respectively, and these have provided a valuable resource for the scientific community.
Journal: Nature, 2020 Jul DOI: 10.1038/s41586-020-2449-8 PMID: 32728248.0 PMCID: PMC7410827
243. Molecular Transducers of Physical Activity Consortium (MoTrPAC): Mapping the Dynamic Responses to Exercise.
Sanford, Nogiec, Lindholm, Adkins, Amar, Dasari, Drugan, Fernandez, Radom-Aizik, Schenk, Snyder, Tracy, Vanderboom, Trappe, Walsh
Exercise provides a robust physiological stimulus that evokes cross-talk among multiple tissues that when repeated regularly (i.e., training) improves physiological capacity, benefits numerous organ systems, and decreases the risk for premature mortality. However, a gap remains in identifying the detailed molecular signals induced by exercise that benefits health and prevents disease. The Molecular Transducers of Physical Activity Consortium (MoTrPAC) was established to address this gap and generate a molecular map of exercise. Preclinical and clinical studies will examine the systemic effects of endurance and resistance exercise across a range of ages and fitness levels by molecular probing of multiple tissues before and after acute and chronic exercise. From this multi-omic and bioinformatic analysis, a molecular map of exercise will be established. Altogether, MoTrPAC will provide a public database that is expected to enhance our understanding of the health benefits of exercise and to provide insight into how physical activity mitigates disease.
Journal: Cell, 2020 Jun 25 DOI: 10.1016/j.cell.2020.06.004 PMID: 32589957.0 PMCID: PMC8800485
242. Analysis of Genetically Regulated Gene Expression Identifies a Prefrontal PTSD Gene, SNRNP35, Specific to Military Cohorts.
Huckins, Chatzinakos, Breen, Hartmann, Klengel, da, Dobbyn, Girdhar, Hoffman, Klengel, Logue, Lori, Maihofer, Morrison, Nguyen, Park, Ruderfer, Sloofman, van, Baker, Chen, Cox, Duncan, Geyer, Glatt, Im, Risbrough, Smoller, Stein, Yehuda, Liberzon, Koenen, Jovanovic, Kellis, Miller, Bacanu, Nievergelt, Buxbaum, Sklar, Ressler, Stahl, Daskalakis
To reveal post-traumatic stress disorder (PTSD) genetic risk influences on tissue-specific gene expression, we use brain and non-brain transcriptomic imputation. We impute genetically regulated gene expression (GReX) in 29,539 PTSD cases and 166,145 controls from 70 ancestry-specific cohorts and identify 18 significant GReX-PTSD associations corresponding to specific tissue-gene pairs. The results suggest substantial genetic heterogeneity based on ancestry, cohort type (military versus civilian), and sex. Two study-wide significant PTSD associations are identified in European and military European cohorts; ZNF140 is predicted to be upregulated in whole blood, and SNRNP35 is predicted to be downregulated in dorsolateral prefrontal cortex, respectively. In peripheral leukocytes from 175 marines, the observed PTSD differential gene expression correlates with the predicted differences for these individuals, and deployment stress produces glucocorticoid-regulated expression changes that include downregulation of both ZNF140 and SNRNP35. SNRNP35 knockdown in cells validates its functional role in U12-intron splicing. Finally, exogenous glucocorticoids in mice downregulate prefrontal Snrnp35 expression.
Journal: Cell Reports, 2020 Jun 2 DOI: 10.1016/j.celrep.2020.107716 PMID: 32492425.0 PMCID: PMC7359754
241. Reconstruction of the human blood-brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes.
Blanchard, Bula, Davila-Velderrain, Akay, Zhu, Frank, Victor, Bonner, Mathys, Lin, Ko, Bennett, Cam, Kellis, Tsai
In Alzheimer's disease, amyloid deposits along the brain vasculature lead to a condition known as cerebral amyloid angiopathy (CAA), which impairs blood-brain barrier (BBB) function and accelerates cognitive degeneration. Apolipoprotein (APOE4) is the strongest risk factor for CAA, yet the mechanisms underlying this genetic susceptibility are unknown. Here we developed an induced pluripotent stem cell-based three-dimensional model that recapitulates anatomical and physiological properties of the human BBB in vitro. Similarly to CAA, our in vitro BBB displayed significantly more amyloid accumulation in APOE4 compared to APOE3. Combinatorial experiments revealed that dysregulation of calcineurin-nuclear factor of activated T cells (NFAT) signaling and APOE in pericyte-like mural cells induces APOE4-associated CAA pathology. In the human brain, APOE and NFAT are selectively dysregulated in pericytes of APOE4 carriers, and inhibition of calcineurin-NFAT signaling reduces APOE4-associated CAA pathology in vitro and in vivo. Our study reveals the role of pericytes in APOE4-mediated CAA and highlights calcineurin-NFAT signaling as a therapeutic target in CAA and Alzheimer's disease.
Journal: Nature Medicine, 2020 Jun DOI: 10.1038/s41591-020-0886-4 PMID: 32514169.0 PMCID: PMC7704032
240. Interleukin-6 deficiency exacerbates Huntington's disease model phenotypes.
Wertz, Pineda, Lee, Kulicke, Kellis, Heiman
Huntington's disease (HD) is an incurable neurodegenerative disorder caused by CAG trinucleotide expansions in the huntingtin gene. Markers of both systemic and CNS immune activation and inflammation have been widely noted in HD and mouse models of HD. In particular, elevation of the pro-inflammatory cytokine interleukin-6 (IL-6) is the earliest reported marker of immune activation in HD, and this elevation has been suggested to contribute to HD pathogenesis. To test the hypothesis that IL-6 deficiency would be protective against the effects of mutant huntingtin, we generated R6/2 HD model mice that lacked IL-6. Contrary to our prediction, IL-6 deficiency exacerbated HD-model associated behavioral phenotypes. Single nuclear RNA Sequencing (snRNA-seq) analysis of striatal cell types revealed that IL-6 deficiency led to the dysregulation of various genes associated with synaptic function, as well as the BDNF receptor Ntrk2. These data suggest that IL-6 deficiency exacerbates the effects of mutant huntingtin through dysregulation of genes of known relevance to HD pathobiology in striatal neurons, and further suggest that modulation of IL-6 to a level that promotes proper regulation of genes associated with synaptic function may hold promise as an HD therapeutic target.
Journal: Molecular Neurodegeneration, 2020 May 24 DOI: 10.1186/s13024-020-00379-3 PMID: 32448329.0 PMCID: PMC7247164
239. Inferring multimodal latent topics from electronic health records.
Li, Nair, Lu, Wen, Wang, Dehaghi, Miao, Liu, Ordog, Biernacka, Ryu, Olson, Frye, Liu, Guo, Marelli, Ahuja, Davila-Velderrain, Kellis
Electronic health records (EHR) are rich heterogeneous collections of patient health information, whose broad adoption provides clinicians and researchers unprecedented opportunities for health informatics, disease-risk prediction, actionable clinical recommendations, and precision medicine. However, EHRs present several modeling challenges, including highly sparse data matrices, noisy irregular clinical notes, arbitrary biases in billing code assignment, diagnosis-driven lab tests, and heterogeneous data types. To address these challenges, we present MixEHR, a multi-view Bayesian topic model. We demonstrate MixEHR on MIMIC-III, Mayo Clinic Bipolar Disorder, and Quebec Congenital Heart Disease EHR datasets. Qualitatively, MixEHR disease topics reveal meaningful combinations of clinical features across heterogeneous data types. Quantitatively, we observe superior prediction accuracy of diagnostic codes and lab test imputations compared to the state-of-art methods. We leverage the inferred patient topic mixtures to classify target diseases and predict mortality of patients in critical conditions. In all comparison, MixEHR confers competitive performance and reveals meaningful disease-related topics.
Journal: Nature Communications, 2020 May 21 DOI: 10.1038/s41467-020-16378-3 PMID: 32439869.0 PMCID: PMC7242436
238. Genome-wide In Vivo CNS Screening Identifies Genes that Modify CNS Neuronal Survival and mHTT Toxicity.
Wertz, Mitchem, Pineda, Hachigian, Lee, Lau, Powers, Kulicke, Madan, Colic, Therrien, Vernon, Beja-Glasser, Hegde, Gao, Kellis, Hart, Doench, Heiman
Unbiased in vivo genome-wide genetic screening is a powerful approach to elucidate new molecular mechanisms, but such screening has not been possible to perform in the mammalian central nervous system (CNS). Here, we report the results of the first genome-wide genetic screens in the CNS using both short hairpin RNA (shRNA) and CRISPR libraries. Our screens identify many classes of CNS neuronal essential genes and demonstrate that CNS neurons are particularly sensitive not only to perturbations to synaptic processes but also autophagy, proteostasis, mRNA processing, and mitochondrial function. These results reveal a molecular logic for the common implication of these pathways across multiple neurodegenerative diseases. To further identify disease-relevant genetic modifiers, we applied our screening approach to two mouse models of Huntington's disease (HD). Top mutant huntingtin toxicity modifier genes included several Nme genes and several genes involved in methylation-dependent chromatin silencing and dopamine signaling, results that reveal new HD therapeutic target pathways.
Journal: Neuron, 2020 Apr 8 DOI: 10.1016/j.neuron.2020.01.004 PMID: 32004439.0 PMCID: PMC7181458
237. Publisher Correction: Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries.
Zekavat, Ruotsalainen, Handsaker, Alver, Bloom, Poterba, Seed, Ernst, Chaffin, Engreitz, Peloso, Manichaikul, Yang, Ryan, Fu, Johnson, Tsai, Budoff, Vasan, Cupples, Rotter, Rich, Post, Mitchell, Correa, Metspalu, Wilson, Salomaa, Kellis, Daly, Neale, McCarroll, Surakka, Esko, Ganna, Ripatti, Kathiresan, Natarajan
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
Journal: Nature Communications, 2020 Apr 1 DOI: 10.1038/s41467-020-15236-6 PMID: 32238811.0 PMCID: PMC7113276
236. Evidence for a novel overlapping coding sequence in POLG initiated at a CUG start codon.
Khan, Jungreis, Wright, Mudge, Choudhary, Firth, Kellis
POLG, located on nuclear chromosome 15, encodes the DNA polymerase gamma(Pol gamma). Pol gamma is responsible for the replication and repair of mitochondrial DNA (mtDNA). Pol gamma is the only DNA polymerase found in mitochondria for most animal cells. Mutations in POLG are the most common single-gene cause of diseases of mitochondria and have been mapped over the coding region of the POLG ORF. RESULTS: Using PhyloCSF to survey alternative reading frames, we found a conserved coding signature in an alternative frame in exons 2 and 3 of POLG, herein referred to as ORF-Y that arose de novo in placental mammals. Using the synplot2 program, synonymous site conservation was found among mammals in the region of the POLG ORF that is overlapped by ORF-Y. Ribosome profiling data revealed that ORF-Y is translated and that initiation likely occurs at a CUG codon. Inspection of an alignment of mammalian sequences containing ORF-Y revealed that the CUG codon has a strong initiation context and that a well-conserved predicted RNA stem-loop begins 14 nucleotides downstream. Such features are associated with enhanced initiation at near-cognate non-AUG codons. Reanalysis of the Kim et al. (2014) draft human proteome dataset yielded two unique peptides that map unambiguously to ORF-Y. An additional conserved uORF, herein referred to as ORF-Z, was also found in exon 2 of POLG. Lastly, we surveyed Clinvar variants that are synonymous with respect to the POLG ORF and found that most of these variants cause amino acid changes in ORF-Y or ORF-Z. CONCLUSIONS: We provide evidence for a novel coding sequence, ORF-Y, that overlaps the POLG ORF. Ribosome profiling and mass spectrometry data show that ORF-Y is expressed. PhyloCSF and synplot2 analysis show that ORF-Y is subject to strong purifying selection. An abundance of disease-correlated mutations that map to exons 2 and 3 of POLG but also affect ORF-Y provides potential clinical significance to this finding.
Journal: Bmc Genetics, 2020 Mar 6 DOI: 10.1186/s12863-020-0828-7 PMID: 32138667.0 PMCID: PMC7059407
235. Few SINEs of life: Alu elements have little evidence for biological relevance despite elevated translation.
Martinez-Gomez, Abascal, Jungreis, Pozo, Kellis, Mudge, Tress
Transposable elements colonize genomes and with time may end up being incorporated into functional regions. SINE Alu elements, which appeared in the primate lineage, are ubiquitous in the human genome and more than a thousand overlap annotated coding exons. Although almost all Alu-derived coding exons appear to be in alternative transcripts, they have been incorporated into the main coding transcript in at least 11 genes. The extent to which Alu regions are incorporated into functional proteins is unclear, but we detected reliable peptide evidence to support the translation to protein of 33 Alu-derived exons. All but one of the Alu elements for which we detected peptides were frame-preserving and there was proportionally seven times more peptide evidence for Alu elements as for other primate exons. Despite this strong evidence for translation to protein we found no evidence of selection, either from cross species alignments or human population variation data, among these Alu-derived exons. Overall, our results confirm that SINE Alu elements have contributed to the expansion of the human proteome, and this contribution appears to be stronger than might be expected over such a relatively short evolutionary timeframe. Despite this, the biological relevance of these modifications remains open to question.
Journal: Nar Genomics And Bioinformatics, 2020 Mar DOI: 10.1093/nargab/lqz023 PMID: 31886458.0 PMCID: PMC6924539
234. The landscape of viral associations in human cancers.
Zapatka, Borozan, Brewer, Iskar, Grundhoff, Alawi, Desai, Sultmann, Moch, Cooper, Eils, Ferretti, Lichter
Here, as part of the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium, for which whole-genome and-for a subset-whole-transcriptome sequencing data from 2,658 cancers across 38 tumor types was aggregated, we systematically investigated potential viral pathogens using a consensus approach that integrated three independent pipelines. Viruses were detected in 382 genome and 68 transcriptome datasets. We found a high prevalence of known tumor-associated viruses such as Epstein-Barr virus (EBV), hepatitis B virus (HBV) and human papilloma virus (HPV; for example, HPV16 or HPV18). The study revealed significant exclusivity of HPV and driver mutations in head-and-neck cancer and the association of HPV with APOBEC mutational signatures, which suggests that impaired antiviral defense is a driving force in cervical, bladder and head-and-neck carcinoma. For HBV, HPV16, HPV18 and adeno-associated virus-2 (AAV2), viral integration was associated with local variations in genomic copy numbers. Integrations at the TERT promoter were associated with high telomerase expression evidently activating this tumor-driving process. High levels of endogenous retrovirus (ERV1) expression were linked to a worse survival outcome in patients with kidney cancer.
Journal: Nature Genetics, 2020 Mar DOI: 10.1038/s41588-019-0558-9 PMID: 32025001.0 PMCID: PMC8076016
233. Comprehensive molecular characterization of mitochondrial genomes in human cancers.
Yuan, Ju, Kim, Li, Wang, Yoon, Yang, Martincorena, Creighton, Weinstein, Xu, Han, Kim, Nakagawa, Park, Campbell, Liang
Mitochondria are essential cellular organelles that play critical roles in cancer. Here, as part of the International Cancer Genome Consortium/The Cancer Genome Atlas Pan-Cancer Analysis of Whole Genomes Consortium, which aggregated whole-genome sequencing data from 2,658 cancers across 38 tumor types, we performed a multidimensional, integrated characterization of mitochondrial genomes and related RNA sequencing data. Our analysis presents the most definitive mutational landscape of mitochondrial genomes and identifies several hypermutated cases. Truncating mutations are markedly enriched in kidney, colorectal and thyroid cancers, suggesting oncogenic effects with the activation of signaling pathways. We find frequent somatic nuclear transfers of mitochondrial DNA, some of which disrupt therapeutic target genes. Mitochondrial copy number varies greatly within and across cancers and correlates with clinical variables. Co-expression analysis highlights the function of mitochondrial genes in oxidative phosphorylation, DNA repair and the cell cycle, and shows their connections with clinically actionable genes. Our study lays a foundation for translating mitochondrial biology into clinical applications.
Journal: Nature Genetics, 2020 Mar DOI: 10.1038/s41588-019-0557-x PMID: 32024997.0 PMCID: PMC7058535
232. Comprehensive analysis of chromothripsis in 2,658 human cancers using whole-genome sequencing.
Cortes-Ciriano, Lee, Xi, Jain, Jung, Yang, Gordenin, Klimczak, Zhang, Pellman, Park
Chromothripsis is a mutational phenomenon characterized by massive, clustered genomic rearrangements that occurs in cancer and other diseases. Recent studies in selected cancer types have suggested that chromothripsis may be more common than initially inferred from low-resolution copy-number data. Here, as part of the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium of the International Cancer Genome Consortium (ICGC) and The Cancer Genome Atlas (TCGA), we analyze patterns of chromothripsis across 2,658 tumors from 38 cancer types using whole-genome sequencing data. We find that chromothripsis events are pervasive across cancers, with a frequency of more than 50% in several cancer types. Whereas canonical chromothripsis profiles display oscillations between two copy-number states, a considerable fraction of events involve multiple chromosomes and additional structural alterations. In addition to non-homologous end joining, we detect signatures of replication-associated processes and templated insertions. Chromothripsis contributes to oncogene amplification and to inactivation of genes such as mismatch-repair-related genes. These findings show that chromothripsis is a major process that drives genome evolution in human cancer.
Journal: Nature Genetics, 2020 Mar DOI: 10.1038/s41588-019-0576-7 PMID: 32025003.0 PMCID: PMC7058534
231. Butler enables rapid cloud-based analysis of thousands of human genomes.
Yakneen, Waszak, Gertz, Korbel
We present Butler, a computational tool that facilitates large-scale genomic analyses on public and academic clouds. Butler includes innovative anomaly detection and self-healing functions that improve the efficiency of data processing and analysis by 43% compared with current approaches. Butler enabled processing of a 725-terabyte cancer genome dataset from the Pan-Cancer Analysis of Whole Genomes (PCAWG) project in a time-efficient and uniform manner.
Journal: Nature Biotechnology, 2020 Mar DOI: 10.1038/s41587-019-0360-3 PMID: 32024987.0 PMCID: PMC7062635
230. Disruption of chromatin folding domains by somatic genomic rearrangements in human cancer.
Akdemir, Le, Chandran, Li, Verhaak, Beroukhim, Campbell, Chin, Dixon, Futreal
Chromatin is folded into successive layers to organize linear DNA. Genes within the same topologically associating domains (TADs) demonstrate similar expression and histone-modification profiles, and boundaries separating different domains have important roles in reinforcing the stability of these features. Indeed, domain disruptions in human cancers can lead to misregulation of gene expression. However, the frequency of domain disruptions in human cancers remains unclear. Here, as part of the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium of the International Cancer Genome Consortium (ICGC) and The Cancer Genome Atlas (TCGA), which aggregated whole-genome sequencing data from 2,658 cancers across 38 tumor types, we analyzed 288,457 somatic structural variations (SVs) to understand the distributions and effects of SVs across TADs. Notably, SVs can lead to the fusion of discrete TADs, and complex rearrangements markedly change chromatin folding maps in the cancer genomes. Notably, only 14% of the boundary deletions resulted in a change in expression in nearby genes of more than twofold.
Journal: Nature Genetics, 2020 Mar DOI: 10.1038/s41588-019-0564-y PMID: 32024999.0 PMCID: PMC7058537
229. Pan-cancer analysis of whole genomes identifies driver rearrangements promoted by LINE-1 retrotransposition.
Rodriguez-Martin, Alvarez, Baez-Ortega, Zamora, Supek, Demeulemeester, Santamarina, Ju, Temes, Garcia-Souto, Detering, Li, Rodriguez-Castro, Dueso-Barroso, Bruzos, Dentro, Blanco, Contino, Ardeljan, Tojo, Roberts, Zumalave, Edwards, Weischenfeldt, Puiggros, Chong, Chen, Lee, Wala, Raine, Butler, Waszak, Navarro, Schumacher, Monlong, Maura, Bolli, Bourque, Gerstein, Park, Wedge, Beroukhim, Torrents, Korbel, Martincorena, Fitzgerald, Van, Kazazian, Burns, Campbell, Tubio
About half of all cancers have somatic integrations of retrotransposons. Here, to characterize their role in oncogenesis, we analyzed the patterns and mechanisms of somatic retrotransposition in 2,954 cancer genomes from 38 histological cancer subtypes within the framework of the Pan-Cancer Analysis of Whole Genomes (PCAWG) project. We identified 19,166 somatically acquired retrotransposition events, which affected 35% of samples and spanned a range of event types. Long interspersed nuclear element (LINE-1; L1 hereafter) insertions emerged as the first most frequent type of somatic structural variation in esophageal adenocarcinoma, and the second most frequent in head-and-neck and colorectal cancers. Aberrant L1 integrations can delete megabase-scale regions of a chromosome, which sometimes leads to the removal of tumor-suppressor genes, and can induce complex translocations and large-scale duplications. Somatic retrotranspositions can also initiate breakage-fusion-bridge cycles, leading to high-level amplification of oncogenes. These observations illuminate a relevant role of L1 retrotransposition in remodeling the cancer genome, with potential implications for the development of human tumors.
Journal: Nature Genetics, 2020 Mar DOI: 10.1038/s41588-019-0562-0 PMID: 32024998.0 PMCID: PMC7058536
228. Pathway and network analysis of more than 2500 whole cancer genomes.
Reyna, Haan, Paczkowska, Verbeke, Vazquez, Kahraman, Pulido-Tamayo, Barenboim, Wadi, Dhingra, Shrestha, Getz, Lawrence, Pedersen, Rubin, Wheeler, Brunak, Izarzugaza, Khurana, Marchal, von, Sahinalp, Valencia, Reimand, Stuart, Raphael
The catalog of cancer driver mutations in protein-coding genes has greatly expanded in the past decade. However, non-coding cancer driver mutations are less well-characterized and only a handful of recurrent non-coding mutations, most notably TERT promoter mutations, have been reported. Here, as part of the ICGC/TCGA Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium, which aggregated whole genome sequencing data from 2658 cancer across 38 tumor types, we perform multi-faceted pathway and network analyses of non-coding mutations across 2583 whole cancer genomes from 27 tumor types compiled by the ICGC/TCGA PCAWG project that was motivated by the success of pathway and network analyses in prioritizing rare mutations in protein-coding genes. While few non-coding genomic elements are recurrently mutated in this cohort, we identify 93 genes harboring non-coding mutations that cluster into several modules of interacting proteins. Among these are promoter mutations associated with reduced mRNA expression in TP53, TLE4, and TCF4. We find that biological processes had variable proportions of coding and non-coding mutations, with chromatin remodeling and proliferation pathways altered primarily by coding mutations, while developmental pathways, including Wnt and Notch, altered by both coding and non-coding mutations. RNA splicing is primarily altered by non-coding mutations in this cohort, and samples containing non-coding mutations in well-known RNA splicing factors exhibit similar gene expression signatures as samples with coding mutations in these genes. These analyses contribute a new repertoire of possible cancer genes and mechanisms that are altered by non-coding mutations and offer insights into additional cancer vulnerabilities that can be investigated for potential therapeutic treatments.
Journal: Nature Communications, 2020 Feb 5 DOI: 10.1038/s41467-020-14367-0 PMID: 32024854.0 PMCID: PMC7002574
227. A deep learning system accurately classifies primary and metastatic cancers using passenger mutation patterns.
Jiao, Atwal, Polak, Karlic, Cuppen, Danyi, de, van, Lolkema, Steeghs, Getz, Morris, Stein
In cancer, the primary tumour's organ of origin and histopathology are the strongest determinants of its clinical behaviour, but in 3% of cases a patient presents with a metastatic tumour and no obvious primary. Here, as part of the ICGC/TCGA Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium, we train a deep learning classifier to predict cancer type based on patterns of somatic passenger mutations detected in whole genome sequencing (WGS) of 2606 tumours representing 24 common cancer types produced by the PCAWG Consortium. Our classifier achieves an accuracy of 91% on held-out tumor samples and 88% and 83% respectively on independent primary and metastatic samples, roughly double the accuracy of trained pathologists when presented with a metastatic tumour without knowledge of the primary. Surprisingly, adding information on driver mutations reduced accuracy. Our results have clinical applicability, underscore how patterns of somatic passenger mutations encode the state of the cell of origin, and can inform future strategies to detect the source of circulating tumour DNA.
Journal: Nature Communications, 2020 Feb 5 DOI: 10.1038/s41467-019-13825-8 PMID: 32024849.0 PMCID: PMC7002586
226. Cancer LncRNA Census reveals evidence for deep functional conservation of long noncoding RNAs in tumorigenesis.
Carlevaro-Fita, Lanzos, Feuerbach, Hong, Mas-Ponte, Pedersen, Johnson
Long non-coding RNAs (lncRNAs) are a growing focus of cancer genomics studies, creating the need for a resource of lncRNAs with validated cancer roles. Furthermore, it remains debated whether mutated lncRNAs can drive tumorigenesis, and whether such functions could be conserved during evolution. Here, as part of the ICGC/TCGA Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium, we introduce the Cancer LncRNA Census (CLC), a compilation of 122 GENCODE lncRNAs with causal roles in cancer phenotypes. In contrast to existing databases, CLC requires strong functional or genetic evidence. CLC genes are enriched amongst driver genes predicted from somatic mutations, and display characteristic genomic features. Strikingly, CLC genes are enriched for driver mutations from unbiased, genome-wide transposon-mutagenesis screens in mice. We identified 10 tumour-causing mutations in orthologues of 8 lncRNAs, including LINC-PINT and NEAT1, but not MALAT1. Thus CLC represents a dataset of high-confidence cancer lncRNAs. Mutagenesis maps are a novel means for identifying deeply-conserved roles of lncRNAs in tumorigenesis.
Journal: Communications Biology, 2020 Feb 5 DOI: 10.1038/s42003-019-0741-7 PMID: 32024996.0 PMCID: PMC7002399
225. Integrative pathway enrichment analysis of multivariate omics data.
Paczkowska, Barenboim, Sintupisut, Fox, Zhu, Abd-Rabbo, Mee, Boutros, Reimand
Multi-omics datasets represent distinct aspects of the central dogma of molecular biology. Such high-dimensional molecular profiles pose challenges to data interpretation and hypothesis generation. ActivePathways is an integrative method that discovers significantly enriched pathways across multiple datasets using statistical data fusion, rationalizes contributing evidence and highlights associated genes. As part of the ICGC/TCGA Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium, which aggregated whole genome sequencing data from 2658 cancers across 38 tumor types, we integrated genes with coding and non-coding mutations and revealed frequently mutated pathways and additional cancer genes with infrequent mutations. We also analyzed prognostic molecular pathways by integrating genomic and transcriptomic features of 1780 breast cancers and highlighted associations with immune response and anti-apoptotic signaling. Integration of ChIP-seq and RNA-seq data for master regulators of the Hippo pathway across normal human tissues identified processes of tissue regeneration and stem cell regulation. ActivePathways is a versatile method that improves systems-level understanding of cellular organization in health and disease through integration of multiple molecular datasets and pathway annotations.
Journal: Nature Communications, 2020 Feb 5 DOI: 10.1038/s41467-019-13983-9 PMID: 32024846.0 PMCID: PMC7002665
224. High-coverage whole-genome analysis of 1220 cancers reveals hundreds of genes deregulated by rearrangement-mediated cis-regulatory alterations.
Zhang, Chen, Fonseca, He, Fujita, Nakagawa, Zhang, Brazma, Creighton
The impact of somatic structural variants (SVs) on gene expression in cancer is largely unknown. Here, as part of the ICGC/TCGA Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium, which aggregated whole-genome sequencing data and RNA sequencing from a common set of 1220 cancer cases, we report hundreds of genes for which the presence within 100 kb of an SV breakpoint associates with altered expression. For the majority of these genes, expression increases rather than decreases with corresponding breakpoint events. Up-regulated cancer-associated genes impacted by this phenomenon include TERT, MDM2, CDK4, ERBB2, CD274, PDCD1LG2, and IGF2. TERT-associated breakpoints involve ~3% of cases, most frequently in liver biliary, melanoma, sarcoma, stomach, and kidney cancers. SVs associated with up-regulation of PD1 and PDL1 genes involve ~1% of non-amplified cases. For many genes, SVs are significantly associated with increased numbers or greater proximity of enhancer regulatory elements near the gene. DNA methylation near the promoter is often increased with nearby SV breakpoint, which may involve inactivation of repressor elements.
Journal: Nature Communications, 2020 Feb 5 DOI: 10.1038/s41467-019-13885-w PMID: 32024823.0 PMCID: PMC7002524
223. Reconstructing evolutionary trajectories of mutation signature activities in cancer using TrackSig.
Rubanova, Shi, Harrigan, Li, Wintersinger, Sahin, Deshwar, Morris
The type and genomic context of cancer mutations depend on their causes. These causes have been characterized using signatures that represent mutation types that co-occur in the same tumours. However, it remains unclear how mutation processes change during cancer evolution due to the lack of reliable methods to reconstruct evolutionary trajectories of mutational signature activity. Here, as part of the ICGC/TCGA Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium, which aggregated whole-genome sequencing data from 2658 cancers across 38 tumour types, we present TrackSig, a new method that reconstructs these trajectories using optimal, joint segmentation and deconvolution of mutation type and allele frequencies from a single tumour sample. In simulations, we find TrackSig has a 3-5% activity reconstruction error, and 12% false detection rate. It outperforms an aggressive baseline in situations with branching evolution, CNA gain, and neutral mutations. Applied to data from 2658 tumours and 38 cancer types, TrackSig permits pan-cancer insight into evolutionary changes in mutational processes.
Journal: Nature Communications, 2020 Feb 5 DOI: 10.1038/s41467-020-14352-7 PMID: 32024834.0 PMCID: PMC7002414
222. Divergent mutational processes distinguish hypoxic and normoxic tumours.
Bhandari, Li, Bristow, Boutros
Many primary tumours have low levels of molecular oxygen (hypoxia), and hypoxic tumours respond poorly to therapy. Pan-cancer molecular hallmarks of tumour hypoxia remain poorly understood, with limited comprehension of its associations with specific mutational processes, non-coding driver genes and evolutionary features. Here, as part of the ICGC/TCGA Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium, which aggregated whole genome sequencing data from 2658 cancers across 38 tumour types, we quantify hypoxia in 1188 tumours spanning 27 cancer types. Elevated hypoxia associates with increased mutational load across cancer types, irrespective of underlying mutational class. The proportion of mutations attributed to several mutational signatures of unknown aetiology directly associates with the level of hypoxia, suggesting underlying mutational processes for these signatures. At the gene level, driver mutations in TP53, MYC and PTEN are enriched in hypoxic tumours, and mutations in PTEN interact with hypoxia to direct tumour evolutionary trajectories. Overall, hypoxia plays a critical role in shaping the genomic and evolutionary landscapes of cancer.
Journal: Nature Communications, 2020 Feb 5 DOI: 10.1038/s41467-019-14052-x PMID: 32024819.0 PMCID: PMC7002770
221. Combined burden and functional impact tests for cancer driver discovery using DriverPower.
Shuai, Gallinger, Stein
The discovery of driver mutations is one of the key motivations for cancer genome sequencing. Here, as part of the ICGC/TCGA Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium, which aggregated whole genome sequencing data from 2658 cancers across 38 tumour types, we describe DriverPower, a software package that uses mutational burden and functional impact evidence to identify driver mutations in coding and non-coding sites within cancer whole genomes. Using a total of 1373 genomic features derived from public sources, DriverPower's background mutation model explains up to 93% of the regional variance in the mutation rate across multiple tumour types. By incorporating functional impact scores, we are able to further increase the accuracy of driver discovery. Testing across a collection of 2583 cancer genomes from the PCAWG project, DriverPower identifies 217 coding and 95 non-coding driver candidates. Comparing to six published methods used by the PCAWG Drivers and Functional Interpretation Working Group, DriverPower has the highest F1 score for both coding and non-coding driver discovery. This demonstrates that DriverPower is an effective framework for computational driver discovery.
Journal: Nature Communications, 2020 Feb 5 DOI: 10.1038/s41467-019-13929-1 PMID: 32024818.0 PMCID: PMC7002750
220. Genomic footprints of activated telomere maintenance mechanisms in cancer.
Sieverling, Hong, Koser, Ginsbach, Kleinheinz, Hutter, Braun, Cortes-Ciriano, Xi, Kabbe, Park, Eils, Schlesner, Brors, Rippe, Jones, Feuerbach
Cancers require telomere maintenance mechanisms for unlimited replicative potential. They achieve this through TERT activation or alternative telomere lengthening associated with ATRX or DAXX loss. Here, as part of the ICGC/TCGA Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium, we dissect whole-genome sequencing data of over 2500 matched tumor-control samples from 36 different tumor types aggregated within the ICGC/TCGA Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium to characterize the genomic footprints of these mechanisms. While the telomere content of tumors with ATRX or DAXX mutations (ATRX/DAXX(trunc)) is increased, tumors with TERT modifications show a moderate decrease of telomere content. One quarter of all tumor samples contain somatic integrations of telomeric sequences into non-telomeric DNA. This fraction is increased to 80% prevalence in ATRX/DAXX(trunc) tumors, which carry an aberrant telomere variant repeat (TVR) distribution as another genomic marker. The latter feature includes enrichment or depletion of the previously undescribed singleton TVRs TTCGGG and TTTGGG, respectively. Our systematic analysis provides new insight into the recurrent genomic alterations associated with telomere maintenance mechanisms in cancer.
Journal: Nature Communications, 2020 Feb 5 DOI: 10.1038/s41467-019-13824-9 PMID: 32024817.0 PMCID: PMC7002710
219. Inferring structural variant cancer cell fraction.
Cmero, Yuan, Ong, Schroder, Corcoran, Papenfuss, Hovens, Markowetz, Macintyre
We present SVclone, a computational method for inferring the cancer cell fraction of structural variant (SV) breakpoints from whole-genome sequencing data. SVclone accurately determines the variant allele frequencies of both SV breakends, then simultaneously estimates the cancer cell fraction and SV copy number. We assess performance using in silico mixtures of real samples, at known proportions, created from two clonal metastases from the same patient. We find that SVclone's performance is comparable to single-nucleotide variant-based methods, despite having an order of magnitude fewer data points. As part of the Pan-Cancer Analysis of Whole Genomes (PCAWG) consortium, which aggregated whole-genome sequencing data from 2658 cancers across 38 tumour types, we use SVclone to reveal a subset of liver, ovarian and pancreatic cancers with subclonally enriched copy-number neutral rearrangements that show decreased overall survival. SVclone enables improved characterisation of SV intra-tumour heterogeneity.
Journal: Nature Communications, 2020 Feb 5 DOI: 10.1038/s41467-020-14351-8 PMID: 32024845.0 PMCID: PMC7002525
218. The evolutionary history of 2,658 cancers.
Gerstung, Jolly, Leshchiner, Dentro, Gonzalez, Rosebrock, Mitchell, Rubanova, Anur, Yu, Tarabichi, Deshwar, Wintersinger, Kleinheinz, Vazquez-Garcia, Haase, Jerman, Sengupta, Macintyre, Malikic, Donmez, Livitz, Cmero, Demeulemeester, Schumacher, Fan, Yao, Lee, Schlesner, Boutros, Bowtell, Zhu, Getz, Imielinski, Beroukhim, Sahinalp, Ji, Peifer, Markowetz, Mustonen, Yuan, Wang, Morris, Spellman, Wedge, Van
Cancer develops through a process of somatic evolution(1,2). Sequencing data from a single biopsy represent a snapshot of this process that can reveal the timing of specific genomic aberrations and the changing influence of mutational processes(3). Here, by whole-genome sequencing analysis of 2,658 cancers as part of the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium of the International Cancer Genome Consortium (ICGC) and The Cancer Genome Atlas (TCGA)(4), we reconstruct the life history and evolution of mutational processes and driver mutation sequences of 38 types of cancer. Early oncogenesis is characterized by mutations in a constrained set of driver genes, and specific copy number gains, such as trisomy 7 in glioblastoma and isochromosome 17q in medulloblastoma. The mutational spectrum changes significantly throughout tumour evolution in 40% of samples. A nearly fourfold diversification of driver genes and increased genomic instability are features of later stages. Copy number alterations often occur in mitotic crises, and lead to simultaneous gains of chromosomal segments. Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer, and highlight opportunities for early cancer detection.
Journal: Nature, 2020 Feb DOI: 10.1038/s41586-019-1907-7 PMID: 32025013.0 PMCID: PMC7054212
217. Genomic basis for RNA alterations in cancer.
Calabrese, Davidson, Demircioglu, Fonseca, He, Kahles, Lehmann, Liu, Shiraishi, Soulette, Urban, Greger, Li, Liu, Perry, Xiang, Zhang, Zhang, Bailey, Erkek, Hoadley, Hou, Huska, Kilpinen, Korbel, Marin, Markowski, Nandi, Pan-Hammarstrom, Pedamallu, Siebert, Stark, Su, Tan, Waszak, Yung, Zhu, Awadalla, Creighton, Meyerson, Ouellette, Wu, Yang, Brazma, Brooks, Goke, Ratsch, Schwarz, Stegle, Zhang
Transcript alterations often result from somatic changes in cancer genomes(1). Various forms of RNA alterations have been described in cancer, including overexpression(2), altered splicing(3) and gene fusions(4); however, it is difficult to attribute these to underlying genomic changes owing to heterogeneity among patients and tumour types, and the relatively small cohorts of patients for whom samples have been analysed by both transcriptome and whole-genome sequencing. Here we present, to our knowledge, the most comprehensive catalogue of cancer-associated gene alterations to date, obtained by characterizing tumour transcriptomes from 1,188 donors of the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium of the International Cancer Genome Consortium (ICGC) and The Cancer Genome Atlas (TCGA)(5). Using matched whole-genome sequencing data, we associated several categories of RNA alterations with germline and somatic DNA alterations, and identified probable genetic mechanisms. Somatic copy-number alterations were the major drivers of variations in total gene and allele-specific expression. We identified 649 associations of somatic single-nucleotide variants with gene expression in cis, of which 68.4% involved associations with flanking non-coding regions of the gene. We found 1,900 splicing alterations associated with somatic mutations, including the formation of exons within introns in proximity to Alu elements. In addition, 82% of gene fusions were associated with structural variants, including 75 of a new class, termed 'bridged' fusions, in which a third genomic location bridges two genes. We observed transcriptomic alteration signatures that differ between cancer types and have associations with variations in DNA mutational signatures. This compendium of RNA alterations in the genomic context provides a rich resource for identifying genes and mechanisms that are functionally implicated in cancer.
Journal: Nature, 2020 Feb DOI: 10.1038/s41586-020-1970-0 PMID: 32025019.0 PMCID: PMC7054216
216. Analyses of non-coding somatic drivers in 2,658 cancer whole genomes.
Rheinbay, Nielsen, Abascal, Wala, Shapira, Tiao, Hornshoj, Hess, Juul, Lin, Feuerbach, Sabarinathan, Madsen, Kim, Mularoni, Shuai, Lanzos, Herrmann, Maruvka, Shen, Amin, Bandopadhayay, Bertl, Boroevich, Busanovich, Carlevaro-Fita, Chakravarty, Chan, Craft, Dhingra, Diamanti, Fonseca, Gonzalez-Perez, Guo, Hamilton, Haradhvala, Hong, Isaev, Johnson, Juul, Kahles, Kahraman, Kim, Komorowski, Kumar, Kumar, Lee, Lehmann, Li, Liu, Lochovsky, Park, Pich, Roberts, Saksena, Schumacher, Sidiropoulos, Sieverling, Sinnott-Armstrong, Stewart, Tamborero, Tubio, Umer, Uuskula-Reimand, Wadelius, Wadi, Yao, Zhang, Zhang, Haber, Hobolth, Imielinski, Kellis, Lawrence, von, Nakagawa, Raphael, Rubin, Sander, Stein, Stuart, Tsunoda, Wheeler, Johnson, Reimand, Gerstein, Khurana, Campbell, Lopez-Bigas, Weischenfeldt, Beroukhim, Martincorena, Pedersen, Getz
The discovery of drivers of cancer has traditionally focused on protein-coding genes(1-4). Here we present analyses of driver point mutations and structural variants in non-coding regions across 2,658 genomes from the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium(5) of the International Cancer Genome Consortium (ICGC) and The Cancer Genome Atlas (TCGA). For point mutations, we developed a statistically rigorous strategy for combining significance levels from multiple methods of driver discovery that overcomes the limitations of individual methods. For structural variants, we present two methods of driver discovery, and identify regions that are significantly affected by recurrent breakpoints and recurrent somatic juxtapositions. Our analyses confirm previously reported drivers(6,7), raise doubts about others and identify novel candidates, including point mutations in the 5' region of TP53, in the 3' untranslated regions of NFKBIZ and TOB1, focal deletions in BRD4 and rearrangements in the loci of AKR1C genes. We show that although point mutations and structural variants that drive cancer are less frequent in non-coding genes and regulatory sequences than in protein-coding genes, additional examples of these drivers will be found as more cancer genomes become available.
Journal: Nature, 2020 Feb DOI: 10.1038/s41586-020-1965-x PMID: 32025015.0 PMCID: PMC7054214
215. Patterns of somatic structural variation in human cancer genomes.
Li, Roberts, Wala, Shapira, Schumacher, Kumar, Khurana, Waszak, Korbel, Haber, Imielinski, Weischenfeldt, Beroukhim, Campbell
A key mutational process in cancer is structural variation, in which rearrangements delete, amplify or reorder genomic segments that range in size from kilobases to whole chromosomes(1-7). Here we develop methods to group, classify and describe somatic structural variants, using data from the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium of the International Cancer Genome Consortium (ICGC) and The Cancer Genome Atlas (TCGA), which aggregated whole-genome sequencing data from 2,658 cancers across 38 tumour types(8). Sixteen signatures of structural variation emerged. Deletions have a multimodal size distribution, assort unevenly across tumour types and patients, are enriched in late-replicating regions and correlate with inversions. Tandem duplications also have a multimodal size distribution, but are enriched in early-replicating regions-as are unbalanced translocations. Replication-based mechanisms of rearrangement generate varied chromosomal structures with low-level copy-number gains and frequent inverted rearrangements. One prominent structure consists of 2-7 templates copied from distinct regions of the genome strung together within one locus. Such cycles of templated insertions correlate with tandem duplications, and-in liver cancer-frequently activate the telomerase gene TERT. A wide variety of rearrangement processes are active in cancer, which generate complex configurations of the genome upon which selection can act.
Journal: Nature, 2020 Feb DOI: 10.1038/s41586-019-1913-9 PMID: 32025012.0 PMCID: PMC7025897
214. Pan-cancer analysis of whole genomes.
The ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium
Cancer is driven by genetic change, and the advent of massively parallel sequencing has enabled systematic documentation of this variation at the whole-genome scale(1-3). Here we report the integrative analysis of 2,658 whole-cancer genomes and their matching normal tissues across 38 tumour types from the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium of the International Cancer Genome Consortium (ICGC) and The Cancer Genome Atlas (TCGA). We describe the generation of the PCAWG resource, facilitated by international data sharing using compute clouds. On average, cancer genomes contained 4-5 driver mutations when combining coding and non-coding genomic elements; however, in around 5% of cases no drivers were identified, suggesting that cancer driver discovery is not yet complete. Chromothripsis, in which many clustered structural variants arise in a single catastrophic event, is frequently an early event in tumour evolution; in acral melanoma, for example, these events precede most somatic point mutations and affect several cancer-associated genes simultaneously. Cancers with abnormal telomere maintenance often originate from tissues with low replicative activity and show several mechanisms of preventing telomere attrition to critical levels. Common and rare germline variants affect patterns of somatic mutation, including point mutations, structural variants and somatic retrotransposition. A collection of papers from the PCAWG Consortium describes non-coding mutations that drive cancer beyond those in the TERT promoter(4); identifies new signatures of mutational processes that cause base substitutions, small insertions and deletions and structural variation(5,6); analyses timings and patterns of tumour evolution(7); describes the diverse transcriptional consequences of somatic mutation on splicing, expression levels, fusion genes and promoter activity(8,9); and evaluates a range of more-specialized features of cancer genomes(8,10-18).
Journal: Nature, 2020 Feb DOI: 10.1038/s41586-020-1969-6 PMID: 32025007.0 PMCID: PMC7025898
213. The repertoire of mutational signatures in human cancer.
Alexandrov, Kim, Haradhvala, Huang, Tian, Wu, Boot, Covington, Gordenin, Bergstrom, Islam, Lopez-Bigas, Klimczak, McPherson, Morganella, Sabarinathan, Wheeler, Mustonen, Getz, Rozen, Stratton
Somatic mutations in cancer genomes are caused by multiple mutational processes, each of which generates a characteristic mutational signature(1). Here, as part of the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium(2) of the International Cancer Genome Consortium (ICGC) and The Cancer Genome Atlas (TCGA), we characterized mutational signatures using 84,729,690 somatic mutations from 4,645 whole-genome and 19,184 exome sequences that encompass most types of cancer. We identified 49 single-base-substitution, 11 doublet-base-substitution, 4 clustered-base-substitution and 17 small insertion-and-deletion signatures. The substantial size of our dataset, compared with previous analyses(3-15), enabled the discovery of new signatures, the separation of overlapping signatures and the decomposition of signatures into components that may represent associated-but distinct-DNA damage, repair and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogues of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA-maintenance processes. However, many signatures are of unknown cause. This analysis provides a systematic perspective on the repertoire of mutational processes that contribute to the development of human cancer.
Journal: Nature, 2020 Feb DOI: 10.1038/s41586-020-1943-3 PMID: 32025018.0 PMCID: PMC7054213
212. Human Primordial Germ Cells Are Specified from Lineage-Primed Progenitors.
Chen, Sun, Hou, Kim, Faith, Aslanyan, Tao, Zheng, Fu, Liu, Kellis, Clark
In vitro gametogenesis is the process of making germline cells from human pluripotent stem cells. The foundation of this model is the quality of the first progenitors called primordial germ cells (PGCs), which in vivo are specified during the peri-implantation window of human development. Here, we show that human PGC (hPGC) specification begins at day 12 post-fertilization. Using single-cell RNA sequencing of hPGC-like cells (hPGCLCs) differentiated from pluripotent stem cells, we discovered that hPGCLC specification involves resetting pluripotency toward a transitional state with shared characteristics between naive and primed pluripotency, followed by differentiation into lineage-primed TFAP2A(+) progenitors. Applying the germline trajectory to TFAP2C mutants reveals that TFAP2C functions in the TFAP2A(+) progenitors upstream of PRDM1 to regulate the expression of SOX17. This serves to protect hPGCLCs from crossing the Weismann's barrier to adopt somatic cell fates and, therefore, is an essential mechanism for successfully initiating in vitro gametogenesis.
Journal: Cell Reports, 2019 Dec 24 DOI: 10.1016/j.celrep.2019.11.083 PMID: 31875561.0 PMCID: PMC6939677
211. Reconstruction of Cell-type-Specific Interactomes at Single-Cell Resolution.
Mohammadi, Davila-Velderrain, Kellis
The human interactome is instrumental in the systems-level study of the cell and the contextualization of disease-associated gene perturbations. However, reference organismal interactomes do not capture the cell-type-specific context in which proteins and modules preferentially act. Here, we introduce SCINET, a computational framework that reconstructs an ensemble of cell-type-specific interactomes by integrating a global, context-independent reference interactome with a single-cell gene-expression profile. SCINET addresses technical challenges of single-cell data by robustly imputing, transforming, and normalizing the initially noisy and sparse expression of data. Inferred cell-level gene interaction probabilities and group-level interaction strengths define cell-type-specific interactomes. We use SCINET to reconstruct and analyze interactomes of the major human brain and immune cell types, revealing specificity and modularity of perturbations associated with neurodegenerative, neuropsychiatric, and autoimmune disorders. We report cell-type interactomes for brain and immune cell types, together with the SCINET package.
Journal: Cell Systems, 2019 Dec 18 DOI: 10.1016/j.cels.2019.10.007 PMID: 31786210.0 PMCID: PMC6943823
210. Discovery of high-confidence human protein-coding genes and exons by whole-genome PhyloCSF helps elucidate 118 GWAS loci.
Mudge, Jungreis, Hunt, Gonzalez, Wright, Kay, Davidson, Fitzgerald, Seal, Tweedie, He, Waterhouse, Li, Bruford, Choudhary, Frankish, Kellis
The most widely appreciated role of DNA is to encode protein, yet the exact portion of the human genome that is translated remains to be ascertained. We previously developed PhyloCSF, a widely used tool to identify evolutionary signatures of protein-coding regions using multispecies genome alignments. Here, we present the first whole-genome PhyloCSF prediction tracks for human, mouse, chicken, fly, worm, and mosquito. We develop a workflow that uses machine learning to predict novel conserved protein-coding regions and efficiently guide their manual curation. We analyze more than 1000 high-scoring human PhyloCSF regions and confidently add 144 conserved protein-coding genes to the GENCODE gene set, as well as additional coding regions within 236 previously annotated protein-coding genes, and 169 pseudogenes, most of them disabled after primates diverged. The majority of these represent new discoveries, including 70 previously undetected protein-coding genes. The novel coding genes are additionally supported by single-nucleotide variant evidence indicative of continued purifying selection in the human lineage, coding-exon splicing evidence from new GENCODE transcripts using next-generation transcriptomic data sets, and mass spectrometry evidence of translation for several new genes. Our discoveries required simultaneous comparative annotation of other vertebrate genomes, which we show is essential to remove spurious ORFs and to distinguish coding from pseudogene regions. Our new coding regions help elucidate disease-associated regions by revealing that 118 GWAS variants previously thought to be noncoding are in fact protein altering. Altogether, our PhyloCSF data sets and algorithms will help researchers seeking to interpret these genomes, while our new annotations present exciting loci for further experimental characterization.
Journal: Genome Research, 2019 Dec DOI: 10.1101/gr.246462.118 PMID: 31537640.0 PMCID: PMC6886504
209. Single-cell transcriptomic atlas of the human retina identifies cell types associated with age-related macular degeneration.
Menon, Mohammadi, Davila-Velderrain, Goods, Cadwell, Xing, Stemmer-Rachamimov, Shalek, Love, Kellis, Hafler
Genome-wide association studies (GWAS) have identified genetic variants associated with age-related macular degeneration (AMD), one of the leading causes of blindness in the elderly. However, it has been challenging to identify the cell types associated with AMD given the genetic complexity of the disease. Here we perform massively parallel single-cell RNA sequencing (scRNA-seq) of human retinas using two independent platforms, and report the first single-cell transcriptomic atlas of the human retina. Using a multi-resolution network-based analysis, we identify all major retinal cell types, and their corresponding gene expression signatures. Heterogeneity is observed within macroglia, suggesting that human retinal glia are more diverse than previously thought. Finally, GWAS-based enrichment analysis identifies glia, vascular cells, and cone photoreceptors to be associated with the risk of AMD. These data provide a detailed analysis of the human retina, and show how scRNA-seq can provide insight into cell types involved in complex, inflammatory genetic diseases.
Journal: Nature Communications, 2019 Oct 25 DOI: 10.1038/s41467-019-12780-8 PMID: 31653841.0 PMCID: PMC6814749
208. Elucidation of Codon Usage Signatures across the Domains of Life.
Novoa, Jungreis, Jaillon, Kellis
Because of the degeneracy of the genetic code, multiple codons are translated into the same amino acid. Despite being "synonymous," these codons are not equally used. Selective pressures are thought to drive the choice among synonymous codons within a genome, while GC content, which is typically attributed to mutational drift, is the major determinant of variation across species. Here, we find that in addition to GC content, interspecies codon usage signatures can also be detected. More specifically, we show that a single amino acid, arginine, is the major contributor to codon usage bias differences across domains of life. We then exploit this finding and show that domain-specific codon bias signatures can be used to classify a given sequence into its corresponding domain of life with high accuracy. We then wondered whether the inclusion of codon usage codon autocorrelation patterns, which reflects the nonrandom distribution of codon occurrences throughout a transcript, might improve the classification performance of our algorithm. However, we find that autocorrelation patterns are not domain-specific, and surprisingly, are unrelated to tRNA reusage, in contrast to previous reports. Instead, our results suggest that codon autocorrelation patterns are a by-product of codon optimality throughout a sequence, where highly expressed genes display autocorrelated "optimal" codons, whereas lowly expressed genes display autocorrelated "nonoptimal" codons.
Journal: Molecular Biology And Evolution, 2019 Oct 1 DOI: 10.1093/molbev/msz124 PMID: 31220870.0 PMCID: PMC6759073
207. Joint profiling of DNA methylation and chromatin architecture in single cells.
Li, Liu, Zhang, Kubo, Yu, Fang, Kellis, Ren
We report a molecular assay, Methyl-HiC, that can simultaneously capture the chromosome conformation and DNA methylome in a cell. Methyl-HiC reveals coordinated DNA methylation status between distal genomic segments that are in spatial proximity in the nucleus, and delineates heterogeneity of both the chromatin architecture and DNA methylome in a mixed population. It enables simultaneous characterization of cell-type-specific chromatin organization and epigenome in complex tissues.
Journal: Nature Methods, 2019 Oct DOI: 10.1038/s41592-019-0502-z PMID: 31384045.0 PMCID: PMC6765429
206. Integrative construction of regulatory region networks in 127 human reference epigenomes by matrix factorization.
Liu, Davila-Velderrain, Zhang, Kellis
Despite large experimental and computational efforts aiming to dissect the mechanisms underlying disease risk, mapping cis-regulatory elements to target genes remains a challenge. Here, we introduce a matrix factorization framework to integrate physical and functional interaction data of genomic segments. The framework was used to predict a regulatory network of chromatin interaction edges linking more than 20 000 promoters and 1.8 million enhancers across 127 human reference epigenomes, including edges that are present in any of the input datasets. Our network integrates functional evidence of correlated activity patterns from epigenomic data and physical evidence of chromatin interactions. An important contribution of this work is the representation of heterogeneous data with different qualities as networks. We show that the unbiased integration of independent data sources suggestive of regulatory interactions produces meaningful associations supported by existing functional and physical evidence, correlating with expected independent biological features.
Journal: Nucleic Acids Research, 2019 Aug 22 DOI: 10.1093/nar/gkz538 PMID: 31265076.0 PMCID: PMC6698807
205. A high-throughput screening and computation platform for identifying synthetic promoters with enhanced cell-state specificity (SPECS).
Wu, Nissim, Stupp, Pery, Binder-Nissim, Weisinger, Enghuus, Palacios, Humphrey, Zhang, Maria, Kellis, Weiss, Rabkin, Tabach, Lu
Cell state-specific promoters constitute essential tools for basic research and biotechnology because they activate gene expression only under certain biological conditions. Synthetic Promoters with Enhanced Cell-State Specificity (SPECS) can be superior to native ones, but the design of such promoters is challenging and frequently requires gene regulation or transcriptome knowledge that is not readily available. Here, to overcome this challenge, we use a next-generation sequencing approach combined with machine learning to screen a synthetic promoter library with 6107 designs for high-performance SPECS for potentially any cell state. We demonstrate the identification of multiple SPECS that exhibit distinct spatiotemporal activity during the programmed differentiation of induced pluripotent stem cells (iPSCs), as well as SPECS for breast cancer and glioblastoma stem-like cells. We anticipate that this approach could be used to create SPECS for gene therapies that are activated in specific cell states, as well as to study natural transcriptional regulatory networks.
Journal: Nature Communications, 2019 Jun 28 DOI: 10.1038/s41467-019-10912-8 PMID: 31253799.0 PMCID: PMC6599391
204. Rate of brain aging and APOE epsilon4 are synergistic risk factors for Alzheimer's disease.
Glorioso, Pfenning, Lee, Bennett, Sibille, Kellis, Guarente
Advanced age and the APOE epsilon4 allele are the two biggest risk factors for Alzheimer's disease (AD) and declining cognitive function. We describe a universal gauge to measure molecular brain age using transcriptome analysis of four human postmortem cohorts (n = 673, ages 25-97) free of neurological disease. In a fifth cohort of older subjects with or without neurological disease (n = 438, ages 67-108), we show that subjects with brains deviating in the older direction from what would be expected based on chronological age show an increase in AD, Parkinson's disease, and cognitive decline. Strikingly, a younger molecular age (-5 yr than chronological age) protects against AD even in the presence of APOE epsilon4 An established DNA methylation gauge for age correlates well with the transcriptome gauge for determination of molecular age and assigning deviations from the expected. Our results suggest that rapid brain aging and APOE epsilon4 are synergistic risk factors, and interventions that slow aging may substantially reduce risk of neurological disease and decline even in the presence of APOE epsilon4.
Journal: Life Science Alliance, 2019 Jun DOI: 10.26508/lsa.201900303 PMID: 31133613.0 PMCID: PMC6537750
203. Single-cell transcriptomic analysis of Alzheimer's disease.
Mathys, Davila-Velderrain, Peng, Gao, Mohammadi, Young, Menon, He, Abdurrob, Jiang, Martorell, Ransohoff, Hafler, Bennett, Kellis, Tsai
Alzheimer's disease is a pervasive neurodegenerative disorder, the molecular complexity of which remains poorly understood. Here, we analysed 80,660 single-nucleus transcriptomes from the prefrontal cortex of 48 individuals with varying degrees of Alzheimer's disease pathology. Across six major brain cell types, we identified transcriptionally distinct subpopulations, including those associated with pathology and characterized by regulators of myelination, inflammation, and neuron survival. The strongest disease-associated changes appeared early in pathological progression and were highly cell-type specific, whereas genes upregulated at late stages were common across cell types and primarily involved in the global stress response. Notably, we found that female cells were overrepresented in disease-associated subpopulations, and that transcriptional responses were substantially different between sexes in several cell types, including oligodendrocytes. Overall, myelination-related processes were recurrently perturbed in multiple cell types, suggesting that myelination has a key role in Alzheimer's disease pathophysiology. Our single-cell transcriptomic resource provides a blueprint for interrogating the molecular and cellular basis of Alzheimer's disease.
Journal: Nature, 2019 Jun DOI: 10.1038/s41586-019-1195-2 PMID: 31042697.0 PMCID: PMC6865822
202. Challenges in IBD Research: Environmental Triggers.
Ho, Lewis, Mayer, Plevy, Chuang, Rappaport, Croitoru, Korzenik, Krischer, Hyams, Judson, Kellis, Jerrett, Miller, Grant, Shtraizent, Honig, Hurtado-Lorenzo, Wu
Environmental triggers is part of five focus areas of the Challenges in IBD research document, which also includes preclinical human IBD mechanisms, novel technologies, precision medicine and pragmatic clinical research. The Challenges in IBD research document provides a comprehensive overview of current gaps in inflammatory bowel diseases (IBD) research and delivers actionable approaches to address them. It is the result of a multidisciplinary input from scientists, clinicians, patients, and funders, and represents a valuable resource for patient centric research prioritization. In particular, the environmental triggers section is focused on the main research gaps in elucidating causality of environmental factors in IBD. Research gaps were identified in: 1) epidemiology of exposures; 2) identification of signatures of biological response to exposures; and 3) mechanisms of how environmental exposures drive IBD. To address these gaps, the implementation of longitudinal prospective studies to determine disease evolution and identify sub-clinical changes in response to exposures is proposed. This can help define critical windows of vulnerability and risk prediction. In addition, systems biology analysis and in silico modeling were proposed as approaches to integrate the IBD exposome for the identification of biological signatures of response to exposures, and to develop prediction models of the effects of environmental factors in driving disease activity and response to therapy. This research could lead to identification of biomarkers of exposures and new modalities for therapeutic intervention. Finally, hypothesis-driven mechanistic studies to understand gene-environment interactions and to validate causality of priority factors should be performed to determine how environment influences clinical outcomes.
Journal: Inflammatory Bowel Diseases, 2019 May 16 DOI: 10.1093/ibd/izz076 PMID: 31095702.0 PMCID: PMC6787673
201. Corrigendum: Loss of LDAH associated with prostate cancer and hearing loss.
Currall, Chen, Sallari, Cotter, Wong, Robertson, Penney, Lunardi, Reschke, Hickox, Yin, Wong, Fung, Brown, Williamson, Sinnott-Armstrong, Kammin, Ivanov, Zepeda-Mendoza, Shen, Quade, Signoretti, Arnos, Banks, Patsopoulos, Liberman, Kellis, Pandolfi, Morton
The authors would like to apologize for errors the following omission from the Funding section of this article. The statement has been corrected online to include the below: “C.C.M. is supported by the NIHR Manchester Biomedical Research Centre.”
Journal: Human Molecular Genetics, 2019 May 15 DOI: 10.1093/hmg/ddz036 PMID: 31222336.0 PMCID: PMC6494788
200. A gene expression atlas of embryonic neurogenesis in Drosophila reveals complex spatiotemporal regulation of lncRNAs.
McCorkindale, Wahle, Werner, Jungreis, Menzel, Shukla, Abreu, Irizarry, Meyer, Kellis, Zinzen
Cell type specification during early nervous system development in Drosophila melanogaster requires precise regulation of gene expression in time and space. Resolving the programs driving neurogenesis has been a major challenge owing to the complexity and rapidity with which distinct cell populations arise. To resolve the cell type-specific gene expression dynamics in early nervous system development, we have sequenced the transcriptomes of purified neurogenic cell types across consecutive time points covering crucial events in neurogenesis. The resulting gene expression atlas comprises a detailed resource of global transcriptome dynamics that permits systematic analysis of how cells in the nervous system acquire distinct fates. We resolve known gene expression dynamics and uncover novel expression signatures for hundreds of genes among diverse neurogenic cell types, most of which remain unstudied. We also identified a set of conserved long noncoding RNAs (lncRNAs) that are regulated in a tissue-specific manner and exhibit spatiotemporal expression during neurogenesis with exquisite specificity. lncRNA expression is highly dynamic and demarcates specific subpopulations within neurogenic cell types. Our spatiotemporal transcriptome atlas provides a comprehensive resource for investigating the function of coding genes and noncoding RNAs during crucial stages of early neurogenesis.
Journal: Development, 2019 Mar 28 DOI: 10.1242/dev.175265 PMID: 30923056.0 PMCID: PMC6451322
199. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use.
Liu, Jiang, Wedow, Li, Brazel, Chen, Datta, Davila-Velderrain, McGuire, Tian, Zhan, Choquet, Docherty, Faul, Foerster, Fritsche, Gabrielsen, Gordon, Haessler, Hottenga, Huang, Jang, Jansen, Ling, Magi, Matoba, McMahon, Mulas, Orru, Palviainen, Pandit, Reginsson, Skogholt, Smith, Taylor, Turman, Willemsen, Young, Young, Zajac, Zhao, Zhou, Bjornsdottir, Boardman, Boehnke, Boomsma, Chen, Cucca, Davies, Eaton, Ehringer, Esko, Fiorillo, Gillespie, Gudbjartsson, Haller, Harris, Heath, Hewitt, Hickie, Hokanson, Hopfer, Hunter, Iacono, Johnson, Kamatani, Kardia, Keller, Kellis, Kooperberg, Kraft, Krauter, Laakso, Lind, Loukola, Lutz, Madden, Martin, McGue, McQueen, Medland, Metspalu, Mohlke, Nielsen, Okada, Peters, Polderman, Posthuma, Reiner, Rice, Rimm, Rose, Runarsdottir, Stallings, Stancakova, Stefansson, Thai, Tindle, Tyrfingsson, Wall, Weir, Weisner, Whitfield, Winsvold, Yin, Zuccolo, Bierut, Hveem, Lee, Munafo, Saccone, Willer, Cornelis, David, Hinds, Jorgenson, Kaprio, Stitzel, Stefansson, Thorgeirsson, Abecasis, Liu, Vrieze
Tobacco and alcohol use are leading causes of mortality that influence risk for many complex diseases and disorders(1). They are heritable(2,3) and etiologically related(4,5) behaviors that have been resistant to gene discovery efforts(6-11). In sample sizes up to 1.2 million individuals, we discovered 566 genetic variants in 406 loci associated with multiple stages of tobacco use (initiation, cessation, and heaviness) as well as alcohol use, with 150 loci evidencing pleiotropic association. Smoking phenotypes were positively genetically correlated with many health conditions, whereas alcohol use was negatively correlated with these conditions, such that increased genetic risk for alcohol use is associated with lower disease risk. We report evidence for the involvement of many systems in tobacco and alcohol use, including genes involved in nicotinic, dopaminergic, and glutamatergic neurotransmission. The results provide a solid starting point to evaluate the effects of these loci in model organisms and more precise substance use measures.
Journal: Nature Genetics, 2019 Feb DOI: 10.1038/s41588-018-0307-5 PMID: 30643251.0 PMCID: PMC6358542
198. An AR-ERG transcriptional signature defined by long-range chromatin interactomes in prostate cancer cells.
Zhang, Chng, Lingadahalli, Chen, Liu, Do, Cai, Rinaldi, Poh, Li, Sung, Heng, Core, Tan, Ruan, Lis, Kellis, Ruan, Sung, Cheung
The aberrant activities of transcription factors such as the androgen receptor (AR) underpin prostate cancer development. While the AR cis-regulation has been extensively studied in prostate cancer, information pertaining to the spatial architecture of the AR transcriptional circuitry remains limited. In this paper, we propose a novel framework to profile long-range chromatin interactions associated with AR and its collaborative transcription factor, erythroblast transformation-specific related gene (ERG), using chromatin interaction analysis by paired-end tag (ChIA-PET). We identified ERG-associated long-range chromatin interactions as a cooperative component in the AR-associated chromatin interactome, acting in concert to achieve coordinated regulation of a subset of AR target genes. Through multifaceted functional data analysis, we found that AR-ERG interaction hub regions are characterized by distinct functional signatures, including bidirectional transcription and cotranscription factor binding. In addition, cancer-associated long noncoding RNAs were found to be connected near protein-coding genes through AR-ERG looping. Finally, we found strong enrichment of prostate cancer genome-wide association study (GWAS) single nucleotide polymorphisms (SNPs) at AR-ERG co-binding sites participating in chromatin interactions and gene regulation, suggesting GWAS target genes identified from chromatin looping data provide more biologically relevant findings than using the nearest gene approach. Taken together, our results revealed an AR-ERG-centric higher-order chromatin structure that drives coordinated gene expression in prostate cancer progression and the identification of potential target genes for therapeutic intervention.
Journal: Genome Research, 2019 Feb DOI: 10.1101/gr.230243.117 PMID: 30606742.0 PMCID: PMC6360806
197. ncdDetect2: improved models of the site-specific mutation rate in cancer and driver detection with robust significance evaluation.
Juul, Madsen, Guo, Bertl, Hobolth, Kellis, Pedersen
Understanding the mutational processes that act during cancer development is a key topic of cancer biology. Nevertheless, much remains to be learned, as a complex interplay of processes with dependencies on a range of genomic features creates highly heterogeneous cancer genomes. Accurate driver detection relies on unbiased models of the mutation rate that also capture rate variation from uncharacterized sources. RESULTS: Here, we analyse patterns of observed-to-expected mutation counts across 505 whole cancer genomes, and find that genomic features missing from our mutation-rate model likely operate on a megabase length scale. We extend our site-specific model of the mutation rate to include the additional variance from these sources, which leads to robust significance evaluation of candidate cancer drivers. We thus present ncdDetect v.2, with greatly improved cancer driver detection specificity. Finally, we show that ranking candidates by their posterior mean value of their effect sizes offers an equivalent and more computationally efficient alternative to ranking by their P-values. AVAILABILITY AND IMPLEMENTATION: ncdDetect v.2 is implemented as an R-package and is freely available at http://github.com/TobiasMadsen/ncdDetect2. SUPPLEMENTARY INFORMATION: Supplementary data are available at Bioinformatics online.
Journal: Bioinformatics, 2019 Jan 15 DOI: 10.1093/bioinformatics/bty511 PMID: 29945188.0 PMCID: PMC6330011
196. GENCODE reference annotation for the human and mouse genomes.
Frankish, Diekhans, Ferreira, Johnson, Jungreis, Loveland, Mudge, Sisu, Wright, Armstrong, Barnes, Berry, Bignell, Carbonell, Chrast, Cunningham, Di, Donaldson, Fiddes, Garcia, Gonzalez, Grego, Hardy, Hourlier, Hunt, Izuogu, Lagarde, Martin, Martinez, Mohanan, Muir, Navarro, Parker, Pei, Pozo, Ruffier, Schmitt, Stapleton, Suner, Sycheva, Uszczynska-Ratajczak, Xu, Yates, Zerbino, Zhang, Aken, Choudhary, Gerstein, Guigo, Hubbard, Kellis, Paten, Reymond, Tress, Flicek
The accurate identification and description of the genes in the human and mouse genomes is a fundamental requirement for high quality analysis of data informing both genome biology and clinical genomics. Over the last 15 years, the GENCODE consortium has been producing reference quality gene annotations to provide this foundational resource. The GENCODE consortium includes both experimental and computational biology groups who work together to improve and extend the GENCODE gene annotation. Specifically, we generate primary data, create bioinformatics tools and provide analysis to support the work of expert manual gene annotators and automated gene annotation pipelines. In addition, manual and computational annotation workflows use any and all publicly available data and analysis, along with the research literature to identify and characterise gene loci to the highest standard. GENCODE gene annotations are accessible via the Ensembl and UCSC Genome Browsers, the Ensembl FTP site, Ensembl Biomart, Ensembl Perl and REST APIs as well as https://www.gencodegenes.org.
Journal: Nucleic Acids Research, 2019 Jan 8 DOI: 10.1093/nar/gky955 PMID: 30357393.0 PMCID: PMC6323946
195. High-resolution genome-wide functional dissection of transcriptional regulatory regions and nucleotides in human.
Wang, He, Goggin, Saadat, Wang, Sinnott-Armstrong, Claussnitzer, Kellis
Genome-wide epigenomic maps have revealed millions of putative enhancers and promoters, but experimental validation of their function and high-resolution dissection of their driver nucleotides remain limited. Here, we present HiDRA (High-resolution Dissection of Regulatory Activity), a combined experimental and computational method for high-resolution genome-wide testing and dissection of putative regulatory regions. We test ~7 million accessible DNA fragments in a single experiment, by coupling accessible chromatin extraction with self-transcribing episomal reporters (ATAC-STARR-seq). By design, fragments are highly overlapping in densely-sampled accessible regions, enabling us to pinpoint driver regulatory nucleotides by exploiting differences in activity between partially-overlapping fragments using a machine learning model (SHARPR-RE). In GM12878 lymphoblastoid cells, we find ~65,000 regions showing enhancer function, and pinpoint ~13,000 high-resolution driver elements. These are enriched for regulatory motifs, evolutionarily-conserved nucleotides, and disease-associated genetic variants from genome-wide association studies. Overall, HiDRA provides a high-throughput, high-resolution approach for dissecting regulatory regions and driver nucleotides.
Journal: Nature Communications, 2018 Dec 19 DOI: 10.1038/s41467-018-07746-1 PMID: 30568279.0 PMCID: PMC6300699
194. Loss of LDAH associated with prostate cancer and hearing loss.
Currall, Chen, Sallari, Cotter, Wong, Robertson, Penney, Lunardi, Reschke, Hickox, Yin, Wong, Fung, Brown, Williamson, Sinnott-Armstrong, Kammin, Ivanov, Zepeda-Mendoza, Shen, Quade, Signoretti, Arnos, Banks, Patsopoulos, Liberman, Kellis, Pandolfi, Morton
Great strides in gene discovery have been made using a multitude of methods to associate phenotypes with genetic variants, but there still remains a substantial gap between observed symptoms and identified genetic defects. Herein, we use the convergence of various genetic and genomic techniques to investigate the underpinnings of a constellation of phenotypes that include prostate cancer (PCa) and sensorineural hearing loss (SNHL) in a human subject. Through interrogation of the subject's de novo, germline, balanced chromosomal translocation, we first identify a correlation between his disorders and a poorly annotated gene known as lipid droplet associated hydrolase (LDAH). Using data repositories of both germline and somatic variants, we identify convergent genomic evidence that substantiates a correlation between loss of LDAH and PCa. This correlation is validated through both in vitro and in vivo models that show loss of LDAH results in increased risk of PCa and, to a lesser extent, SNHL. By leveraging convergent evidence in emerging genomic data, we hypothesize that loss of LDAH is involved in PCa and other phenotypes observed in support of a genotype-phenotype association in an n-of-one human subject.
Journal: Human Molecular Genetics, 2018 Dec 15 DOI: 10.1093/hmg/ddy310 PMID: 30169630.0 PMCID: PMC6276829
193. Corrigendum: Loose ends: almost one in five human genes still have unresolved coding status.
Abascal, Juan, Jungreis, Kellis, Martinez, Rigau, Rodriguez, Vazquez, Tress
Seventeen years after the sequencing of the human genome, the human proteome is still under revision. One in eight of the 22 210 coding genes listed by the Ensembl/GENCODE, RefSeq and UniProtKB reference databases are annotated differently across the three sets. We have carried out an in-depth investigation on the 2764 genes classified as coding by one or more sets of manual curators and not coding by others. Data from large-scale genetic variation analyses suggests that most are not under protein-like purifying selection and so are unlikely to code for functional proteins. A further 1470 genes annotated as coding in all three reference sets have characteristics that are typical of non-coding genes or pseudogenes. These potential non-coding genes also appear to be undergoing neutral evolution and have considerably less supporting transcript and protein evidence than other coding genes. We believe that the three reference databases currently overestimate the number of human coding genes by at least 2000, complicating and adding noise to large-scale biomedical experiments. Determining which potential non-coding genes do not code for proteins is a difficult but vitally important task since the human reference proteome is a fundamental pillar of most basic research and supports almost all large-scale biomedical projects.
Journal: Nucleic Acids Research, 2018 Dec 14 DOI: 10.1093/nar/gky1146 PMID: 30395326.0 PMCID: PMC6294512
192. Target site specificity and in vivo complexity of the mammalian arginylome.
Wang, Pejaver, Dann, Wolf, Kellis, Huang, Garcia, Radivojac, Kashina
Protein arginylation mediated by arginyltransferase ATE1 is a key regulatory process essential for mammalian embryogenesis, cell migration, and protein regulation. Despite decades of studies, very little is known about the specificity of ATE1-mediated target site recognition. Here, we used in vitro assays and computational analysis to dissect target site specificity of mouse arginyltransferases and gain insights into the complexity of the mammalian arginylome. We found that the four ATE1 isoforms have different, only partially overlapping target site specificity that includes more variability in the target residues than previously believed. Based on all the available data, we generated an algorithm for identifying potential arginylation consensus motif and used this algorithm for global prediction of proteins arginylated in vivo on the N-terminal D and E. Our analysis reveals multiple proteins with potential ATE1 target sites and expand our understanding of the biological complexity of the intracellular arginylome.
Journal: Scientific Reports, 2018 Nov 1 DOI: 10.1038/s41598-018-34639-6 PMID: 30385798.0 PMCID: PMC6212499
191. Allele-specific epigenome maps reveal sequence-dependent stochastic switching at regulatory loci.
Onuchic, Lurie, Carrero, Pawliczek, Patel, Rozowsky, Galeev, Huang, Altshuler, Zhang, Harris, Coarfa, Ashmore, Bertol, Fakhouri, Yu, Kellis, Gerstein, Milosavljevic
To assess the impact of genetic variation in regulatory loci on human health, we constructed a high-resolution map of allelic imbalances in DNA methylation, histone marks, and gene transcription in 71 epigenomes from 36 distinct cell and tissue types from 13 donors. Deep whole-genome bisulfite sequencing of 49 methylomes revealed sequence-dependent CpG methylation imbalances at thousands of heterozygous regulatory loci. Such loci are enriched for stochastic switching, which is defined as random transitions between fully methylated and unmethylated states of DNA. The methylation imbalances at thousands of loci are explainable by different relative frequencies of the methylated and unmethylated states for the two alleles. Further analyses provided a unifying model that links sequence-dependent allelic imbalances of the epigenome, stochastic switching at gene regulatory loci, and disease-associated genetic variation.
Journal: Science, 2018 Sep 28 DOI: 10.1126/science.aar3146 PMID: 30139913.0 PMCID: PMC6198826
190. RANGER-DTL 2.0: rigorous reconstruction of gene-family evolution by duplication, transfer and loss.
Bansal, Kellis, Kordi, Kundu
RANGER-DTL 2.0 is a software program for inferring gene family evolution using Duplication-Transfer-Loss reconciliation. This new software is highly scalable and easy to use, and offers many new features not currently available in any other reconciliation program. RANGER-DTL 2.0 has a particular focus on reconciliation accuracy and can account for many sources of reconciliation uncertainty including uncertain gene tree rooting, gene tree topological uncertainty, multiple optimal reconciliations and alternative event cost assignments. RANGER-DTL 2.0 is open-source and written in C++ and Python. AVAILABILITY AND IMPLEMENTATION: Pre-compiled executables, source code (open-source under GNU GPL) and a detailed manual are freely available from http://compbio.engr.uconn.edu/software/RANGER-DTL/. SUPPLEMENTARY INFORMATION: Supplementary data are available at Bioinformatics online.
Journal: Bioinformatics, 2018 Sep 15 DOI: 10.1093/bioinformatics/bty314 PMID: 29688310.0 PMCID: PMC6137995
189. Publisher Correction: Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries.
Zekavat, Ruotsalainen, Handsaker, Alver, Bloom, Poterba, Seed, Ernst, Chaffin, Engreitz, Peloso, Manichaikul, Yang, Ryan, Fu, Johnson, Tsai, Budoff, Vasan, Cupples, Rotter, Rich, Post, Mitchell, Correa, Metspalu, Wilson, Salomaa, Kellis, Daly, Neale, McCarroll, Surakka, Esko, Ganna, Ripatti, Kathiresan, Natarajan
The original version of this article contained an error in the name of the author Ramachandran S. Vasan, which was incorrectly given as Vasan S. Ramachandran. This has now been corrected in both the PDF and HTML versions of the article.
Journal: Nature Communications, 2018 Aug 23 DOI: 10.1038/s41467-018-05975-y PMID: 30140049.0 PMCID: PMC6107495
188. Deep-coverage whole genome sequences and blood lipids among 16,324 individuals.
Natarajan, Peloso, Zekavat, Montasser, Ganna, Chaffin, Khera, Zhou, Bloom, Engreitz, Ernst, O'Connell, Ruotsalainen, Alver, Manichaikul, Johnson, Perry, Poterba, Seed, Surakka, Esko, Ripatti, Salomaa, Correa, Vasan, Kellis, Neale, Lander, Abecasis, Mitchell, Rich, Wilson, Cupples, Rotter, Willer, Kathiresan
Large-scale deep-coverage whole-genome sequencing (WGS) is now feasible and offers potential advantages for locus discovery. We perform WGS in 16,324 participants from four ancestries at mean depth >29X and analyze genotypes with four quantitative traits-plasma total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol, and triglycerides. Common variant association yields known loci except for few variants previously poorly imputed. Rare coding variant association yields known Mendelian dyslipidemia genes but rare non-coding variant association detects no signals. A high 2M-SNP LDL-C polygenic score (top 5th percentile) confers similar effect size to a monogenic mutation (~30 mg/dl higher for each); however, among those with severe hypercholesterolemia, 23% have a high polygenic score and only 2% carry a monogenic mutation. At these sample sizes and for these phenotypes, the incremental value of WGS for discovery is limited but WGS permits simultaneous assessment of monogenic and polygenic models to severe hypercholesterolemia.
Journal: Nature Communications, 2018 Aug 23 DOI: 10.1038/s41467-018-05747-8 PMID: 30140000.0 PMCID: PMC6107638
187. Loose ends: almost one in five human genes still have unresolved coding status.
Abascal, Juan, Jungreis, Kellis, Martinez, Rigau, Rodriguez, Vazquez, Tress
Seventeen years after the sequencing of the human genome, the human proteome is still under revision. One in eight of the 22 210 coding genes listed by the Ensembl/GENCODE, RefSeq and UniProtKB reference databases are annotated differently across the three sets. We have carried out an in-depth investigation on the 2764 genes classified as coding by one or more sets of manual curators and not coding by others. Data from large-scale genetic variation analyses suggests that most are not under protein-like purifying selection and so are unlikely to code for functional proteins. A further 1470 genes annotated as coding in all three reference sets have characteristics that are typical of non-coding genes or pseudogenes. These potential non-coding genes also appear to be undergoing neutral evolution and have considerably less supporting transcript and protein evidence than other coding genes. We believe that the three reference databases currently overestimate the number of human coding genes by at least 2000, complicating and adding noise to large-scale biomedical experiments. Determining which potential non-coding genes do not code for proteins is a difficult but vitally important task since the human reference proteome is a fundamental pillar of most basic research and supports almost all large-scale biomedical projects.
Journal: Nucleic Acids Research, 2018 Aug 21 DOI: 10.1093/nar/gky587 PMID: 29982784.0 PMCID: PMC6101605
186. Analyses of mRNA structure dynamics identify embryonic gene regulatory programs.
Beaudoin, Novoa, Vejnar, Yartseva, Takacs, Kellis, Giraldez
RNA folding plays a crucial role in RNA function. However, knowledge of the global structure of the transcriptome is limited to cellular systems at steady state, thus hindering the understanding of RNA structure dynamics during biological transitions and how it influences gene function. Here, we characterized mRNA structure dynamics during zebrafish development. We observed that on a global level, translation guides structure rather than structure guiding translation. We detected a decrease in structure in translated regions and identified the ribosome as a major remodeler of RNA structure in vivo. In contrast, we found that 3' untranslated regions (UTRs) form highly folded structures in vivo, which can affect gene expression by modulating microRNA activity. Furthermore, dynamic 3'-UTR structures contain RNA-decay elements, such as the regulatory elements in nanog and ccna1, two genes encoding key maternal factors orchestrating the maternal-to-zygotic transition. These results reveal a central role of RNA structure dynamics in gene regulatory programs.
Journal: Nature Structural & Molecular Biology, 2018 Aug DOI: 10.1038/s41594-018-0091-z PMID: 30061596.0 PMCID: PMC6690192
185. Publisher Correction: N(6)-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications.
Wang, Li, Yue, Wang, Kumar, Wechsler-Reya, Zhang, Ogawa, Kellis, Duester, Zhao
In the version of this article initially published online, there were errors in URLs for www.southernbiotech.com, appearing in Methods sections "m6A dot-blot" and "Western blot analysis." The first two URLs should be https://www.southernbiotech.com/?catno=4030-05&type=Polyclonal#&panel1-1 and the third should be https://www.southernbiotech.com/?catno=6170-05&type=Polyclonal. In addition, some Methods URLs for bioz.com, www.abcam.com and www.sysy.com were printed correctly but not properly linked. The errors have been corrected in the PDF and HTML versions of this article.
Journal: Nature +J187Neuroscience, 2018 Aug DOI: 10.1038/s41593-018-0169-2 PMID: 29880878.0
184. Chromatin Accessibility Impacts Transcriptional Reprogramming in Oocytes.
Miyamoto, Nguyen, Allen, Jullien, Kumar, Otani, Bradshaw, Livesey, Kellis, Gurdon
Oocytes have a remarkable ability to reactivate silenced genes in somatic cells. However, it is not clear how the chromatin architecture of somatic cells affects this transcriptional reprogramming. Here, we investigated the relationship between the chromatin opening and transcriptional activation. We reveal changes in chromatin accessibility and their relevance to transcriptional reprogramming after transplantation of somatic nuclei into Xenopus oocytes. Genes that are silenced, but have pre-existing open transcription start sites in donor cells, are prone to be activated after nuclear transfer, suggesting that the chromatin signature of somatic nuclei influences transcriptional reprogramming. There are also activated genes associated with new open chromatin sites, and transcription factors in oocytes play an important role in transcriptional reprogramming from such genes. Finally, we show that genes resistant to reprogramming are associated with closed chromatin configurations. We conclude that chromatin accessibility is a central factor for successful transcriptional reprogramming in oocytes.
Journal: Cell Reports, 2018 Jul 10 DOI: 10.1016/j.celrep.2018.06.030 PMID: 29996092.0 PMCID: PMC6057489
183. Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries.
Zekavat, Ruotsalainen, Handsaker, Alver, Bloom, Poterba, Seed, Ernst, Chaffin, Engreitz, Peloso, Manichaikul, Yang, Ryan, Fu, Johnson, Tsai, Budoff, Vasan, Cupples, Rotter, Rich, Post, Mitchell, Correa, Metspalu, Wilson, Salomaa, Kellis, Daly, Neale, McCarroll, Surakka, Esko, Ganna, Ripatti, Kathiresan, Natarajan
Lipoprotein(a), Lp(a), is a modified low-density lipoprotein particle that contains apolipoprotein(a), encoded by LPA, and is a highly heritable, causal risk factor for cardiovascular diseases that varies in concentrations across ancestries. Here, we use deep-coverage whole genome sequencing in 8392 individuals of European and African ancestry to discover and interpret both single-nucleotide variants and copy number (CN) variation associated with Lp(a). We observe that genetic determinants between Europeans and Africans have several unique determinants. The common variant rs12740374 associated with Lp(a) cholesterol is an eQTL for SORT1 and independent of LDL cholesterol. Observed associations of aggregates of rare non-coding variants are largely explained by LPA structural variation, namely the LPA kringle IV 2 (KIV2)-CN. Finally, we find that LPA risk genotypes confer greater relative risk for incident atherosclerotic cardiovascular diseases compared to directly measured Lp(a), and are significantly associated with measures of subclinical atherosclerosis in African Americans.
Journal: Nature Communications, 2018 Jul 4 DOI: 10.1038/s41467-018-04668-w PMID: 29973585.0 PMCID: PMC6031652
182. Using an atlas of gene regulation across 44 human tissues to inform complex disease- and trait-associated variation.
Gamazon, Segre, van, Wen, Xi, Hormozdiari, Ongen, Konkashbaev, Derks, Aguet, Quan, Nicolae, Eskin, Kellis, Getz, McCarthy, Dermitzakis, Cox, Ardlie
We apply integrative approaches to expression quantitative loci (eQTLs) from 44 tissues from the Genotype-Tissue Expression project and genome-wide association study data. About 60% of known trait-associated loci are in linkage disequilibrium with a cis-eQTL, over half of which were not found in previous large-scale whole blood studies. Applying polygenic analyses to metabolic, cardiovascular, anthropometric, autoimmune, and neurodegenerative traits, we find that eQTLs are significantly enriched for trait associations in relevant pathogenic tissues and explain a substantial proportion of the heritability (40-80%). For most traits, tissue-shared eQTLs underlie a greater proportion of trait associations, although tissue-specific eQTLs have a greater contribution to some traits, such as blood pressure. By integrating information from biological pathways with eQTL target genes and applying a gene-based approach, we validate previously implicated causal genes and pathways, and propose new variant and gene associations for several complex traits, which we replicate in the UK BioBank and BioVU.
Journal: Nature Genetics, 2018 Jul DOI: 10.1038/s41588-018-0154-4 PMID: 29955180.0 PMCID: PMC6248311
181. Stop codon readthrough generates a C-terminally extended variant of the human vitamin D receptor with reduced calcitriol response.
Loughran, Jungreis, Tzani, Power, Dmitriev, Ivanov, Kellis, Atkins
Although stop codon readthrough is used extensively by viruses to expand their gene expression, verified instances of mammalian readthrough have only recently been uncovered by systems biology and comparative genomics approaches. Previously, our analysis of conserved protein coding signatures that extend beyond annotated stop codons predicted stop codon readthrough of several mammalian genes, all of which have been validated experimentally. Four mRNAs display highly efficient stop codon readthrough, and these mRNAs have a UGA stop codon immediately followed by CUAG (UGA_CUAG) that is conserved throughout vertebrates. Extending on the identification of this readthrough motif, we here investigated stop codon readthrough, using tissue culture reporter assays, for all previously untested human genes containing UGA_CUAG. The readthrough efficiency of the annotated stop codon for the sequence encoding vitamin D receptor (VDR) was 6.7%. It was the highest of those tested but all showed notable levels of readthrough. The VDR is a member of the nuclear receptor superfamily of ligand-inducible transcription factors, and it binds its major ligand, calcitriol, via its C-terminal ligand-binding domain. Readthrough of the annotated VDR mRNA results in a 67 amino acid-long C-terminal extension that generates a VDR proteoform named VDRx. VDRx may form homodimers and heterodimers with VDR but, compared with VDR, VDRx displayed a reduced transcriptional response to calcitriol even in the presence of its partner retinoid X receptor.
Journal: The Journal Of Biological Chemistry, 2018 Mar 23 DOI: 10.1074/jbc.M117.818526 PMID: 29386352.0 PMCID: PMC5868278
180. N(6)-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications.
Wang, Li, Yue, Wang, Kumar, Wechsler-Reya, Zhang, Ogawa, Kellis, Duester, Zhao
Internal N(6)-methyladenosine (m(6)A) modification is widespread in messenger RNAs (mRNAs) and is catalyzed by heterodimers of methyltransferase-like protein 3 (Mettl3) and Mettl14. To understand the role of m(6)A in development, we deleted Mettl14 in embryonic neural stem cells (NSCs) in a mouse model. Phenotypically, NSCs lacking Mettl14 displayed markedly decreased proliferation and premature differentiation, suggesting that m(6)A modification enhances NSC self-renewal. Decreases in the NSC pool led to a decreased number of late-born neurons during cortical neurogenesis. Mechanistically, we discovered a genome-wide increase in specific histone modifications in Mettl14 knockout versus control NSCs. These changes correlated with altered gene expression and observed cellular phenotypes, suggesting functional significance of altered histone modifications in knockout cells. Finally, we found that m(6)A regulates histone modification in part by destabilizing transcripts that encode histone-modifying enzymes. Our results suggest an essential role for m(6)A in development and reveal m(6)A-regulated histone modifications as a previously unknown mechanism of gene regulation in mammalian cells.
Journal: Nature Neuroscience, 2018 Feb DOI: 10.1038/s41593-017-0057-1 PMID: 29335608.0 PMCID: PMC6317335
179. Pan-cancer screen for mutations in non-coding elements with conservation and cancer specificity reveals correlations with expression and survival.
Hornshoj, Nielsen, Sinnott-Armstrong, Switnicki, Juul, Madsen, Sallari, Kellis, Orntoft, Hobolth, Pedersen
Cancer develops by accumulation of somatic driver mutations, which impact cellular function. Mutations in non-coding regulatory regions can now be studied genome-wide and further characterized by correlation with gene expression and clinical outcome to identify driver candidates. Using a new two-stage procedure, called ncDriver, we first screened 507 ICGC whole-genomes from 10 cancer types for non-coding elements, in which mutations are both recurrent and have elevated conservation or cancer specificity. This identified 160 significant non-coding elements, including the TERT promoter, a well-known non-coding driver element, as well as elements associated with known cancer genes and regulatory genes (e.g., PAX5, TOX3, PCF11, MAPRE3). However, in some significant elements, mutations appear to stem from localized mutational processes rather than recurrent positive selection in some cases. To further characterize the driver potential of the identified elements and shortlist candidates, we identified elements where presence of mutations correlated significantly with expression levels (e.g., TERT and CDH10) and survival (e.g., CDH9 and CDH10) in an independent set of 505 TCGA whole-genome samples. In a larger pan-cancer set of 4128 TCGA exomes with expression profiling, we identified mutational correlation with expression for additional elements (e.g., near GATA3, CDC6, ZNF217, and CTCF transcription factor binding sites). Survival analysis further pointed to MIR122, a known marker of poor prognosis in liver cancer. In conclusion, the screen for significant mutation patterns coupled with correlative mutational analysis identified new individual driver candidates and suggest that some non-coding mutations recurrently affect expression and play a role in cancer development.
Journal: Npj Genomic Medicine, 2018 DOI: 10.1038/s41525-017-0040-5 PMID: 29354286.0 PMCID: PMC5765157
178. Chromatin-state discovery and genome annotation with ChromHMM.
Ernst, Kellis
Noncoding DNA regions have central roles in human biology, evolution, and disease. ChromHMM helps to annotate the noncoding genome using epigenomic information across one or multiple cell types. It combines multiple genome-wide epigenomic maps, and uses combinatorial and spatial mark patterns to infer a complete annotation for each cell type. ChromHMM learns chromatin-state signatures using a multivariate hidden Markov model (HMM) that explicitly models the combinatorial presence or absence of each mark. ChromHMM uses these signatures to generate a genome-wide annotation for each cell type by calculating the most probable state for each genomic segment. ChromHMM provides an automated enrichment analysis of the resulting annotations to facilitate the functional interpretations of each chromatin state. ChromHMM is distinguished by its modeling emphasis on combinations of marks, its tight integration with downstream functional enrichment analyses, its speed, and its ease of use. Chromatin states are learned, annotations are produced, and enrichments are computed within 1 d.
Journal: Nature Protocols, 2017 Dec DOI: 10.1038/nprot.2017.124 PMID: 29120462.0 PMCID: PMC5945550
177. Enhancing GTEx by bridging the gaps between genotype, gene expression, and disease.
The GTEx Consortium
Genetic variants have been associated with myriad molecular phenotypes that provide new insight into the range of mechanisms underlying genetic traits and diseases. Identifying any particular genetic variant's cascade of effects, from molecule to individual, requires assaying multiple layers of molecular complexity. We introduce the Enhancing GTEx (eGTEx) project that extends the GTEx project to combine gene expression with additional intermediate molecular measurements on the same tissues to provide a resource for studying how genetic differences cascade through molecular phenotypes to impact human health.
Journal: Nature Genetics, 2017 Dec DOI: 10.1038/ng.3969 PMID: 29019975.0 PMCID: PMC6636856
176. Co-expression networks reveal the tissue-specific regulation of transcription and splicing.
Saha, Kim, Gewirtz, Jo, Gao, McDowell, Engelhardt, Battle
Gene co-expression networks capture biologically important patterns in gene expression data, enabling functional analyses of genes, discovery of biomarkers, and interpretation of genetic variants. Most network analyses to date have been limited to assessing correlation between total gene expression levels in a single tissue or small sets of tissues. Here, we built networks that additionally capture the regulation of relative isoform abundance and splicing, along with tissue-specific connections unique to each of a diverse set of tissues. We used the Genotype-Tissue Expression (GTEx) project v6 RNA sequencing data across 50 tissues and 449 individuals. First, we developed a framework called Transcriptome-Wide Networks (TWNs) for combining total expression and relative isoform levels into a single sparse network, capturing the interplay between the regulation of splicing and transcription. We built TWNs for 16 tissues and found that hubs in these networks were strongly enriched for splicing and RNA binding genes, demonstrating their utility in unraveling regulation of splicing in the human transcriptome. Next, we used a Bayesian biclustering model that identifies network edges unique to a single tissue to reconstruct Tissue-Specific Networks (TSNs) for 26 distinct tissues and 10 groups of related tissues. Finally, we found genetic variants associated with pairs of adjacent nodes in our networks, supporting the estimated network structures and identifying 20 genetic variants with distant regulatory impact on transcription and splicing. Our networks provide an improved understanding of the complex relationships of the human transcriptome across tissues.
Journal: Genome Research, 2017 Nov DOI: 10.1101/gr.216721.116 PMID: 29021288.0 PMCID: PMC5668942
175. Identifying cis-mediators for trans-eQTLs across many human tissues using genomic mediation analysis.
Yang, Wang, Pierce, Chen
The impact of inherited genetic variation on gene expression in humans is well-established. The majority of known expression quantitative trait loci (eQTLs) impact expression of local genes (cis-eQTLs). More research is needed to identify effects of genetic variation on distant genes (trans-eQTLs) and understand their biological mechanisms. One common trans-eQTLs mechanism is "mediation" by a local (cis) transcript. Thus, mediation analysis can be applied to genome-wide SNP and expression data in order to identify transcripts that are "cis-mediators" of trans-eQTLs, including those "cis-hubs" involved in regulation of many trans-genes. Identifying such mediators helps us understand regulatory networks and suggests biological mechanisms underlying trans-eQTLs, both of which are relevant for understanding susceptibility to complex diseases. The multitissue expression data from the Genotype-Tissue Expression (GTEx) program provides a unique opportunity to study cis-mediation across human tissue types. However, the presence of complex hidden confounding effects in biological systems can make mediation analyses challenging and prone to confounding bias, particularly when conducted among diverse samples. To address this problem, we propose a new method: Genomic Mediation analysis with Adaptive Confounding adjustment (GMAC). It enables the search of a very large pool of variables, and adaptively selects potential confounding variables for each mediation test. Analyses of simulated data and GTEx data demonstrate that the adaptive selection of confounders by GMAC improves the power and precision of mediation analysis. Application of GMAC to GTEx data provides new insights into the observed patterns of cis-hubs and trans-eQTL regulation across tissue types.
Journal: Genome Research, 2017 Nov DOI: 10.1101/gr.216754.116 PMID: 29021290.0 PMCID: PMC5668943
174. Evidence of reduced recombination rate in human regulatory domains.
Liu, Sarkar, Kheradpour, Ernst, Kellis
Recombination rate is non-uniformly distributed across the human genome. The variation of recombination rate at both fine and large scales cannot be fully explained by DNA sequences alone. Epigenetic factors, particularly DNA methylation, have recently been proposed to influence the variation in recombination rate. RESULTS: We study the relationship between recombination rate and gene regulatory domains, defined by a gene and its linked control elements. We define these links using expression quantitative trait loci (eQTLs), methylation quantitative trait loci (meQTLs), chromatin conformation from publicly available datasets (Hi-C and ChIA-PET), and correlated activity links that we infer across cell types. Each link type shows a "recombination rate valley" of significantly reduced recombination rate compared to matched control regions. This recombination rate valley is most pronounced for gene regulatory domains of early embryonic development genes, housekeeping genes, and constitutive regulatory elements, which are known to show increased evolutionary constraint across species. Recombination rate valleys show increased DNA methylation, reduced doublestranded break initiation, and increased repair efficiency, specifically in the lineage leading to the germ line. Moreover, by using only the overlap of functional links and DNA methylation in germ cells, we are able to predict the recombination rate with high accuracy. CONCLUSIONS: Our results suggest the existence of a recombination rate valley at regulatory domains and provide a potential molecular mechanism to interpret the interplay between genetic and epigenetic variations.
Journal: Genome Biology, 2017 Oct 20 DOI: 10.1186/s13059-017-1308-x PMID: 29058599.0 PMCID: PMC5651596
173. Genetic effects on gene expression across human tissues.
Battle, Brown, Engelhardt, Montgomery
Characterization of the molecular function of the human genome and its variation across individuals is essential for identifying the cellular mechanisms that underlie human genetic traits and diseases. The Genotype-Tissue Expression (GTEx) project aims to characterize variation in gene expression levels across individuals and diverse tissues of the human body, many of which are not easily accessible. Here we describe genetic effects on gene expression levels across 44 human tissues. We find that local genetic variation affects gene expression levels for the majority of genes, and we further identify inter-chromosomal genetic effects for 93 genes and 112 loci. On the basis of the identified genetic effects, we characterize patterns of tissue specificity, compare local and distal effects, and evaluate the functional properties of the genetic effects. We also demonstrate that multi-tissue, multi-individual data can be used to identify genes and pathways affected by human disease-associated variation, enabling a mechanistic interpretation of gene regulation and the genetic basis of disease.
Journal: Nature, 2017 Oct 11 DOI: 10.1038/nature24277 PMID: 29022597.0 PMCID: PMC5776756
172. Dynamic landscape and regulation of RNA editing in mammals.
Tan, Li, Shanmugam, Piskol, Kohler, Young, Liu, Zhang, Ramaswami, Ariyoshi, Gupte, Keegan, George, Ramu, Huang, Pollina, Leeman, Rustighi, Goh, Chawla, Del, Peltz, Brunet, Conrad, Samuel, O'Connell, Walkley, Nishikura, Li
Adenosine-to-inosine (A-to-I) RNA editing is a conserved post-transcriptional mechanism mediated by ADAR enzymes that diversifies the transcriptome by altering selected nucleotides in RNA molecules. Although many editing sites have recently been discovered, the extent to which most sites are edited and how the editing is regulated in different biological contexts are not fully understood. Here we report dynamic spatiotemporal patterns and new regulators of RNA editing, discovered through an extensive profiling of A-to-I RNA editing in 8,551 human samples (representing 53 body sites from 552 individuals) from the Genotype-Tissue Expression (GTEx) project and in hundreds of other primate and mouse samples. We show that editing levels in non-repetitive coding regions vary more between tissues than editing levels in repetitive regions. Globally, ADAR1 is the primary editor of repetitive sites and ADAR2 is the primary editor of non-repetitive coding sites, whereas the catalytically inactive ADAR3 predominantly acts as an inhibitor of editing. Cross-species analysis of RNA editing in several tissues revealed that species, rather than tissue type, is the primary determinant of editing levels, suggesting stronger cis-directed regulation of RNA editing for most sites, although the small set of conserved coding sites is under stronger trans-regulation. In addition, we curated an extensive set of ADAR1 and ADAR2 targets and showed that many editing sites display distinct tissue-specific regulation by the ADAR enzymes in vivo. Further analysis of the GTEx data revealed several potential regulators of editing, such as AIMP2, which reduces editing in muscles by enhancing the degradation of the ADAR proteins. Collectively, our work provides insights into the complex cis- and trans-regulation of A-to-I editing.
Journal: Nature, 2017 Oct 11 DOI: 10.1038/nature24041 PMID: 29022589.0 PMCID: PMC5723435
171. The impact of rare variation on gene expression across tissues.
Li, Kim, Tsang, Davis, Damani, Chiang, Hess, Zappala, Strober, Scott, Li, Ganna, Bassik, Merker, Hall, Battle, Montgomery
Rare genetic variants are abundant in humans and are expected to contribute to individual disease risk. While genetic association studies have successfully identified common genetic variants associated with susceptibility, these studies are not practical for identifying rare variants. Efforts to distinguish pathogenic variants from benign rare variants have leveraged the genetic code to identify deleterious protein-coding alleles, but no analogous code exists for non-coding variants. Therefore, ascertaining which rare variants have phenotypic effects remains a major challenge. Rare non-coding variants have been associated with extreme gene expression in studies using single tissues, but their effects across tissues are unknown. Here we identify gene expression outliers, or individuals showing extreme expression levels for a particular gene, across 44 human tissues by using combined analyses of whole genomes and multi-tissue RNA-sequencing data from the Genotype-Tissue Expression (GTEx) project v6p release. We find that 58% of underexpression and 28% of overexpression outliers have nearby conserved rare variants compared to 8% of non-outliers. Additionally, we developed RIVER (RNA-informed variant effect on regulation), a Bayesian statistical model that incorporates expression data to predict a regulatory effect for rare variants with higher accuracy than models using genomic annotations alone. Overall, we demonstrate that rare variants contribute to large gene expression changes across tissues and provide an integrative method for interpretation of rare variants in individual genomes.
Journal: Nature, 2017 Oct 11 DOI: 10.1038/nature24267 PMID: 29022581.0 PMCID: PMC5877409
170. Landscape of X chromosome inactivation across human tissues.
Tukiainen, Villani, Yen, Rivas, Marshall, Satija, Aguirre, Gauthier, Fleharty, Kirby, Cummings, Castel, Karczewski, Aguet, Byrnes, Lappalainen, Regev, Ardlie, Hacohen, MacArthur
X chromosome inactivation (XCI) silences transcription from one of the two X chromosomes in female mammalian cells to balance expression dosage between XX females and XY males. XCI is, however, incomplete in humans: up to one-third of X-chromosomal genes are expressed from both the active and inactive X chromosomes (Xa and Xi, respectively) in female cells, with the degree of 'escape' from inactivation varying between genes and individuals. The extent to which XCI is shared between cells and tissues remains poorly characterized, as does the degree to which incomplete XCI manifests as detectable sex differences in gene expression and phenotypic traits. Here we describe a systematic survey of XCI, integrating over 5,500 transcriptomes from 449 individuals spanning 29 tissues from GTEx (v6p release) and 940 single-cell transcriptomes, combined with genomic sequence data. We show that XCI at 683 X-chromosomal genes is generally uniform across human tissues, but identify examples of heterogeneity between tissues, individuals and cells. We show that incomplete XCI affects at least 23% of X-chromosomal genes, identify seven genes that escape XCI with support from multiple lines of evidence and demonstrate that escape from XCI results in sex biases in gene expression, establishing incomplete XCI as a mechanism that is likely to introduce phenotypic diversity. Overall, this updated catalogue of XCI across human tissues helps to increase our understanding of the extent and impact of the incompleteness in the maintenance of XCI.
Journal: Nature, 2017 Oct 11 DOI: 10.1038/nature24265 PMID: 29022598.0 PMCID: PMC5685192
169. Predicting gene expression in massively parallel reporter assays: A comparative study.
Kreimer, Zeng, Edwards, Guo, Tian, Shin, Welch, Wainberg, Mohan, Sinnott-Armstrong, Li, Eraslan, Amin, Tewhey, Sabeti, Goke, Mueller, Kellis, Kundaje, Beer, Keles, Gifford, Yosef
In many human diseases, associated genetic changes tend to occur within noncoding regions, whose effect might be related to transcriptional control. A central goal in human genetics is to understand the function of such noncoding regions: given a region that is statistically associated with changes in gene expression (expression quantitative trait locus [eQTL]), does it in fact play a regulatory role? And if so, how is this role "coded" in its sequence? These questions were the subject of the Critical Assessment of Genome Interpretation eQTL challenge. Participants were given a set of sequences that flank eQTLs in humans and were asked to predict whether these are capable of regulating transcription (as evaluated by massively parallel reporter assays), and whether this capability changes between alternative alleles. Here, we report lessons learned from this community effort. By inspecting predictive properties in isolation, and conducting meta-analysis over the competing methods, we find that using chromatin accessibility and transcription factor binding as features in an ensemble of classifiers or regression models leads to the most accurate results. We then characterize the loci that are harder to predict, putting the spotlight on areas of weakness, which we expect to be the subject of future studies.
Journal: Human Mutation, 2017 Sep DOI: 10.1002/humu.23197 PMID: 28220625.0 PMCID: PMC5560998
168. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk.
Day, Thompson, Helgason, Chasman, Finucane, Sulem, Ruth, Whalen, Sarkar, Albrecht, Altmaier, Amini, Barbieri, Boutin, Campbell, Demerath, Giri, He, Hottenga, Karlsson, Kolcic, Loh, Lunetta, Mangino, Marco, McMahon, Medland, Nolte, Noordam, Nutile, Paternoster, Perjakova, Porcu, Rose, Schraut, Segre, Smith, Stolk, Teumer, Andrulis, Bandinelli, Beckmann, Benitez, Bergmann, Bochud, Boerwinkle, Bojesen, Bolla, Brand, Brauch, Brenner, Broer, Bruning, Buring, Campbell, Catamo, Chanock, Chenevix-Trench, Corre, Couch, Cousminer, Cox, Crisponi, Czene, Davey, de, de, De, Dennis, Devilee, Dos-Santos-Silva, Dunning, Eriksson, Fasching, Fernandez-Rhodes, Ferrucci, Flesch-Janys, Franke, Gabrielson, Gandin, Giles, Grallert, Gudbjartsson, Guenel, Hall, Hallberg, Hamann, Harris, Hartman, Heiss, Hooning, Hopper, Hu, Hunter, Ikram, Im, Jarvelin, Joshi, Karasik, Kellis, Kutalik, LaChance, Lambrechts, Langenberg, Launer, Laven, Lenarduzzi, Li, Lind, Lindstrom, Liu, Luan, Magi, Mannermaa, Mbarek, McCarthy, Meisinger, Meitinger, Menni, Metspalu, Michailidou, Milani, Milne, Montgomery, Mulligan, Nalls, Navarro, Nevanlinna, Nyholt, Oldehinkel, O'Mara, Padmanabhan, Palotie, Pedersen, Peters, Peto, Pharoah, Pouta, Radice, Rahman, Ring, Robino, Rosendaal, Rudan, Rueedi, Ruggiero, Sala, Schmidt, Scott, Shah, Sorice, Southey, Sovio, Stampfer, Steri, Strauch, Tanaka, Tikkanen, Timpson, Traglia, Truong, Tyrer, Uitterlinden, Edwards, Vitart, Volker, Vollenweider, Wang, Widen, van, Willemsen, Winqvist, Wolffenbuttel, Zhao, Zoledziewska, Zygmunt, Alizadeh, Boomsma, Ciullo, Cucca, Esko, Franceschini, Gieger, Gudnason, Hayward, Kraft, Lawlor, Magnusson, Martin, Mook-Kanamori, Nohr, Polasek, Porteous, Price, Ridker, Snieder, Spector, Stockl, Toniolo, Ulivi, Visser, Volzke, Wareham, Wilson, Spurdle, Thorsteindottir, Pollard, Easton, Tung, Chang-Claude, Hinds, Murray, Murabito, Stefansson, Ong, Perry
The timing of puberty is a highly polygenic childhood trait that is epidemiologically associated with various adult diseases. Using 1000 Genomes Project-imputed genotype data in up to approximately 370,000 women, we identify 389 independent signals (P < 5 x 10(-8)) for age at menarche, a milestone in female pubertal development. In Icelandic data, these signals explain approximately 7.4% of the population variance in age at menarche, corresponding to approximately 25% of the estimated heritability. We implicate approximately 250 genes via coding variation or associated expression, demonstrating significant enrichment in neural tissues. Rare variants near the imprinted genes MKRN3 and DLK1 were identified, exhibiting large effects when paternally inherited. Mendelian randomization analyses suggest causal inverse associations, independent of body mass index (BMI), between puberty timing and risks for breast and endometrial cancers in women and prostate cancer in men. In aggregate, our findings highlight the complexity of the genetic regulation of puberty timing and support causal links with cancer susceptibility.
Journal: Nature Genetics, 2017 Jun DOI: 10.1038/ng.3841 PMID: 28436984.0 PMCID: PMC5841952
167. Multi-scale chromatin state annotation using a hierarchical hidden Markov model.
Marco, Meuleman, Huang, Glass, Pinello, Wang, Kellis, Yuan
Chromatin-state analysis is widely applied in the studies of development and diseases. However, existing methods operate at a single length scale, and therefore cannot distinguish large domains from isolated elements of the same type. To overcome this limitation, we present a hierarchical hidden Markov model, diHMM, to systematically annotate chromatin states at multiple length scales. We apply diHMM to analyse a public ChIP-seq data set. diHMM not only accurately captures nucleosome-level information, but identifies domain-level states that vary in nucleosome-level state composition, spatial distribution and functionality. The domain-level states recapitulate known patterns such as super-enhancers, bivalent promoters and Polycomb repressed regions, and identify additional patterns whose biological functions are not yet characterized. By integrating chromatin-state information with gene expression and Hi-C data, we identify context-dependent functions of nucleosome-level states. Thus, diHMM provides a powerful tool for investigating the role of higher-order chromatin structure in gene regulation.
Journal: Nature Communications, 2017 Apr 7 DOI: 10.1038/ncomms15011 PMID: 28387224.0 PMCID: PMC5385569
166. Evolutionary Dynamics of Abundant Stop Codon Readthrough.
Jungreis, Chan, Waterhouse, Fields, Lin, Kellis
Translational stop codon readthrough emerged as a major regulatory mechanism affecting hundreds of genes in animal genomes, based on recent comparative genomics and ribosomal profiling evidence, but its evolutionary properties remain unknown. Here, we leverage comparative genomic evidence across 21 Anopheles mosquitoes to systematically annotate readthrough genes in the malaria vector Anopheles gambiae, and to provide the first study of abundant readthrough evolution, by comparison with 20 Drosophila species. Using improved comparative genomics methods for detecting readthrough, we identify evolutionary signatures of conserved, functional readthrough of 353 stop codons in the malaria vector, Anopheles gambiae, and of 51 additional Drosophila melanogaster stop codons, including several cases of double and triple readthrough and of readthrough of two adjacent stop codons. We find that most differences between the readthrough repertoires of the two species arose from readthrough gain or loss in existing genes, rather than birth of new genes or gene death; that readthrough-associated RNA structures are sometimes gained or lost while readthrough persists; that readthrough is more likely to be lost at TAA and TAG stop codons; and that readthrough is under continued purifying evolutionary selection in mosquito, based on population genetic evidence. We also determine readthrough-associated gene properties that predate readthrough, and identify differences in the characteristic properties of readthrough genes between clades. We estimate more than 600 functional readthrough stop codons in mosquito and 900 in fruit fly, provide evidence of readthrough control of peroxisomal targeting, and refine the phylogenetic extent of abundant readthrough as following divergence from centipede.
Journal: Molecular Biology And Evolution, 2016 Dec DOI: 10.1093/molbev/msw189 PMID: 27604222.0 PMCID: PMC5100048
165. MicroRNA and gene expression changes in unruptured human cerebral aneurysms.
Bekelis, Kerley-Hamilton, Teegarden, Tomlinson, Kuintzle, Simmons, Singer, Roberts, Kellis, Hendrix
The molecular mechanisms behind cerebral aneurysm formation and rupture remain poorly understood. In the past decade, microRNAs (miRNAs) have been shown to be key regulators in a host of biological processes. They are noncoding RNA molecules, approximately 21 nucleotides long, that posttranscriptionally inhibit mRNAs by attenuating protein translation and promoting mRNA degradation. The miRNA and mRNA interactions and expression levels in cerebral aneurysm tissue from human subjects were profiled. METHODS A prospective case-control study was performed on human subjects to characterize the differential expression of mRNA and miRNA in unruptured cerebral aneurysms in comparison with control tissue (healthy superficial temporal arteries [STA]). Ion Torrent was used for deep RNA sequencing. Affymetrix miRNA microarrays were used to analyze miRNA expression, whereas NanoString nCounter technology was used for validation of the identified targets. RESULTS Overall, 7 unruptured cerebral aneurysm and 10 STA specimens were collected. Several differentially expressed genes were identified in aneurysm tissue, with MMP-13 (fold change 7.21) and various collagen genes (COL1A1, COL5A1, COL5A2) being among the most upregulated. In addition, multiple miRNAs were significantly differentially expressed, with miR-21 (fold change 16.97) being the most upregulated, and miR-143-5p (fold change -11.14) being the most downregulated. From these, miR-21, miR-143, and miR-145 had several significantly anticorrelated target genes in the cohort that are associated with smooth muscle cell function, extracellular matrix remodeling, inflammation signaling, and lipid accumulation. All these processes are crucial to the pathophysiology of cerebral aneurysms. CONCLUSIONS This analysis identified differentially expressed genes and miRNAs in unruptured human cerebral aneurysms, suggesting the possibility of a role for miRNAs in aneurysm formation. Further investigation for their importance as therapeutic targets is needed.
Journal: Journal Of Neurosurgery, 2016 Dec DOI: 10.3171/2015.11.JNS151841 PMID: 26918470.0 PMCID: PMC5001931
164. Soft X-Ray Tomography Reveals Gradual Chromatin Compaction and Reorganization during Neurogenesis In Vivo.
Le, Clowney, Magklara, Yen, Markenscoff-Papadimitriou, Colquitt, Myllys, Kellis, Lomvardas, Larabell
The realization that nuclear distribution of DNA, RNA, and proteins differs between cell types and developmental stages suggests that nuclear organization serves regulatory functions. Understanding the logic of nuclear architecture and how it contributes to differentiation and cell fate commitment remains challenging. Here, we use soft X-ray tomography (SXT) to image chromatin organization, distribution, and biophysical properties during neurogenesis in vivo. Our analyses reveal that chromatin with similar biophysical properties forms an elaborate connected network throughout the entire nucleus. Although this interconnectivity is present in every developmental stage, differentiation proceeds with concomitant increase in chromatin compaction and re-distribution of condensed chromatin toward the nuclear core. HP1beta, but not nucleosome spacing or phasing, regulates chromatin rearrangements because it governs both the compaction of chromatin and its interactions with the nuclear envelope. Our experiments introduce SXT as a powerful imaging technology for nuclear architecture.
Journal: Cell Reports, 2016 Nov 15 DOI: 10.1016/j.celrep.2016.10.060 PMID: 27851973.0 PMCID: PMC5135017
163. SwiSpot: modeling riboswitches by spotting out switching sequences.
Barsacchi, Novoa, Kellis, Bechini
Riboswitches are cis-regulatory elements in mRNA, mostly found in Bacteria, which exhibit two main secondary structure conformations. Although one of them prevents the gene from being expressed, the other conformation allows its expression, and this switching process is typically driven by the presence of a specific ligand. Although there are a handful of known riboswitches, our knowledge in this field has been greatly limited due to our inability to identify their alternate structures from their sequences. Indeed, current methods are not able to predict the presence of the two functionally distinct conformations just from the knowledge of the plain RNA nucleotide sequence. Whether this would be possible, for which cases, and what prediction accuracy can be achieved, are currently open questions. RESULTS: Here we show that the two alternate secondary structures of riboswitches can be accurately predicted once the 'switching sequence' of the riboswitch has been properly identified. The proposed SwiSpot approach is capable of identifying the switching sequence inside a putative, complete riboswitch sequence, on the basis of pairing behaviors, which are evaluated on proper sets of configurations. Moreover, it is able to model the switching behavior of riboswitches whose generated ensemble covers both alternate configurations. Beyond structural predictions, the approach can also be paired to homology-based riboswitch searches. AVAILABILITY AND IMPLEMENTATION: SwiSpot software, along with the reference dataset files, is available at: http://www.iet.unipi.it/a.bechini/swispot/Supplementary information: Supplementary data are available at Bioinformatics online. CONTACT: a.bechini@ing.unipi.it.
Journal: Bioinformatics, 2016 Nov 1 DOI: 10.1093/bioinformatics/btw401 PMID: 27378291.0
162. Genome-scale high-resolution mapping of activating and repressive nucleotides in regulatory regions.
Ernst, Melnikov, Zhang, Wang, Rogov, Mikkelsen, Kellis
Massively parallel reporter assays (MPRAs) enable nucleotide-resolution dissection of transcriptional regulatory regions, such as enhancers, but only few regions at a time. Here we present a combined experimental and computational approach, Systematic high-resolution activation and repression profiling with reporter tiling using MPRA (Sharpr-MPRA), that allows high-resolution analysis of thousands of regions simultaneously. Sharpr-MPRA combines dense tiling of overlapping MPRA constructs with a probabilistic graphical model to recognize functional regulatory nucleotides, and to distinguish activating and repressive nucleotides, using their inferred contribution to reporter gene expression. We used Sharpr-MPRA to test 4.6 million nucleotides spanning 15,000 putative regulatory regions tiled at 5-nucleotide resolution in two human cell types. Our results recovered known cell-type-specific regulatory motifs and evolutionarily conserved nucleotides, and distinguished known activating and repressive motifs. Our results also showed that endogenous chromatin state and DNA accessibility are both predictive of regulatory function in reporter assays, identified retroviral elements with activating roles, and uncovered 'attenuator' motifs with repressive roles in active chromatin.
Journal: Nature Biotechnology, 2016 Nov DOI: 10.1038/nbt.3678 PMID: 27701403.0 PMCID: PMC5125825
161. Joint Bayesian inference of risk variants and tissue-specific epigenomic enrichments across multiple complex human diseases.
Li, Kellis
Genome wide association studies (GWAS) provide a powerful approach for uncovering disease-associated variants in human, but fine-mapping the causal variants remains a challenge. This is partly remedied by prioritization of disease-associated variants that overlap GWAS-enriched epigenomic annotations. Here, we introduce a new Bayesian model RiVIERA (Risk Variant Inference using Epigenomic Reference Annotations) for inference of driver variants from summary statistics across multiple traits using hundreds of epigenomic annotations. In simulation, RiVIERA promising power in detecting causal variants and causal annotations, the multi-trait joint inference further improved the detection power. We applied RiVIERA to model the existing GWAS summary statistics of 9 autoimmune diseases and Schizophrenia by jointly harnessing the potential causal enrichments among 848 tissue-specific epigenomics annotations from ENCODE/Roadmap consortium covering 127 cell/tissue types and 8 major epigenomic marks. RiVIERA identified meaningful tissue-specific enrichments for enhancer regions defined by H3K4me1 and H3K27ac for Blood T-Cell specifically in the nine autoimmune diseases and Brain-specific enhancer activities exclusively in Schizophrenia. Moreover, the variants from the 95% credible sets exhibited high conservation and enrichments for GTEx whole-blood eQTLs located within transcription-factor-binding-sites and DNA-hypersensitive-sites. Furthermore, joint modeling the nine immune traits by simultaneously inferring and exploiting the underlying epigenomic correlation between traits further improved the functional enrichments compared to single-trait models.
Journal: Nucleic Acids Research, 2016 Oct 14 DOI: 10.1093/nar/gkw627 PMID: 27407109.0 PMCID: PMC5062982
160. 52 Genetic Loci Influencing Myocardial Mass.
van, van, Verweij, Vogler, Franke, Maurano, Wang, Mateo, Eijgelsheim, Sotoodehnia, Hayward, Sorice, Meirelles, Lyytikainen, Polasek, Tanaka, Arking, Ulivi, Trompet, Muller-Nurasyid, Smith, Dorr, Kerr, Magnani, Del, Zhang, Nolte, Silva, Padmanabhan, Tragante, Esko, Abecasis, Adriaens, Andersen, Barnett, Bis, Bodmer, Buckley, Campbell, Cannon, Chakravarti, Chen, Delitala, Devereux, Doevendans, Dominiczak, Ferrucci, Ford, Gieger, Harris, Haugen, Heinig, Hernandez, Hillege, Hirschhorn, Hofman, Hubner, Hwang, Iorio, Kahonen, Kellis, Kolcic, Kooner, Kooner, Kors, Lakatta, Lage, Launer, Levy, Lundby, Macfarlane, May, Meitinger, Metspalu, Nappo, Naitza, Neph, Nord, Nutile, Okin, Olsen, Oostra, Penninger, Pennacchio, Pers, Perz, Peters, Pinto, Pfeufer, Pilia, Pramstaller, Prins, Raitakari, Raychaudhuri, Rice, Rossin, Rotter, Schafer, Schlessinger, Schmidt, Sehmi, Sillje, Sinagra, Sinner, Slowikowski, Soliman, Spector, Spiering, Stamatoyannopoulos, Stolk, Strauch, Tan, Tarasov, Trinh, Uitterlinden, van, van, van, Viikari, Visscher, Vitart, Volker, Waldenberger, Weichenberger, Westra, Wijmenga, Wolffenbuttel, Yang, Bezzina, Munroe, Snieder, Wright, Rudan, Boyer, Asselbergs, van, Stricker, Psaty, Ciullo, Sanna, Lehtimaki, Wilson, Bandinelli, Alonso, Gasparini, Jukema, Kaab, Gudnason, Felix, Heckbert, de, Newton-Cheh, Hicks, Chambers, Jamshidi, Visel, Christoffels, Isaacs, Samani, de
Myocardial mass is a key determinant of cardiac muscle function and hypertrophy. Myocardial depolarization leading to cardiac muscle contraction is reflected by the amplitude and duration of the QRS complex on the electrocardiogram (ECG). Abnormal QRS amplitude or duration reflect changes in myocardial mass and conduction, and are associated with increased risk of heart failure and death. OBJECTIVES: This meta-analysis sought to gain insights into the genetic determinants of myocardial mass. METHODS: We carried out a genome-wide association meta-analysis of 4 QRS traits in up to 73,518 individuals of European ancestry, followed by extensive biological and functional assessment. RESULTS: We identified 52 genomic loci, of which 32 are novel, that are reliably associated with 1 or more QRS phenotypes at p < 1 x 10(-8). These loci are enriched in regions of open chromatin, histone modifications, and transcription factor binding, suggesting that they represent regions of the genome that are actively transcribed in the human heart. Pathway analyses provided evidence that these loci play a role in cardiac hypertrophy. We further highlighted 67 candidate genes at the identified loci that are preferentially expressed in cardiac tissue and associated with cardiac abnormalities in Drosophila melanogaster and Mus musculus. We validated the regulatory function of a novel variant in the SCN5A/SCN10A locus in vitro and in vivo. CONCLUSIONS: Taken together, our findings provide new insights into genes and biological pathways controlling myocardial mass and may help identify novel therapeutic targets.
Journal: Journal Of The American College Of Cardiology, 2016 Sep 27 DOI: 10.1016/j.jacc.2016.07.729 PMID: 27659466.0 PMCID: PMC5478167
159. Discovery and validation of sub-threshold genome-wide association study loci using epigenomic signatures.
Wang, Tucker, Rizki, Mills, Krijger, de, Subramanian, Bartell, Nguyen, Ye, Leyton-Mange, Dolmatova, van, de, Ellinor, Newton-Cheh, Milan, Kellis, Boyer
Genetic variants identified by genome-wide association studies explain only a modest proportion of heritability, suggesting that meaningful associations lie 'hidden' below current thresholds. Here, we integrate information from association studies with epigenomic maps to demonstrate that enhancers significantly overlap known loci associated with the cardiac QT interval and QRS duration. We apply functional criteria to identify loci associated with QT interval that do not meet genome-wide significance and are missed by existing studies. We demonstrate that these 'sub-threshold' signals represent novel loci, and that epigenomic maps are effective at discriminating true biological signals from noise. We experimentally validate the molecular, gene-regulatory, cellular and organismal phenotypes of these sub-threshold loci, demonstrating that most sub-threshold loci have regulatory consequences and that genetic perturbation of nearby genes causes cardiac phenotypes in mouse. Our work provides a general approach for improving the detection of novel loci associated with complex human traits.
Journal: Elife, 2016 May 10 DOI: 10.7554/eLife.10557 PMID: 27162171.0 PMCID: PMC4862755
158. Discovery of Genetic Variation on Chromosome 5q22 Associated with Mortality in Heart Failure.
Smith, Felix, Morrison, Kalogeropoulos, Trompet, Wilk, Gidlof, Wang, Morley, Mendelson, Joehanes, Ligthart, Shan, Bis, Wang, Sjogren, Ngwa, Brandimarto, Stott, Aguilar, Rice, Sesso, Demissie, Buckley, Taylor, Ford, Yao, Liu, Sotoodehnia, van, Stricker, Kritchevsky, Liu, Gaziano, Hofman, Moravec, Uitterlinden, Kellis, van, Margulies, Dehghan, Levy, Olde, Psaty, Cupples, Jukema, Djousse, Franco, Boerwinkle, Boyer, Newton-Cheh, Butler, Vasan, Cappola, Smith
Failure of the human heart to maintain sufficient output of blood for the demands of the body, heart failure, is a common condition with high mortality even with modern therapeutic alternatives. To identify molecular determinants of mortality in patients with new-onset heart failure, we performed a meta-analysis of genome-wide association studies and follow-up genotyping in independent populations. We identified and replicated an association for a genetic variant on chromosome 5q22 with 36% increased risk of death in subjects with heart failure (rs9885413, P = 2.7x10-9). We provide evidence from reporter gene assays, computational predictions and epigenomic marks that this polymorphism increases activity of an enhancer region active in multiple human tissues. The polymorphism was further reproducibly associated with a DNA methylation signature in whole blood (P = 4.5x10-40) that also associated with allergic sensitization and expression in blood of the cytokine TSLP (P = 1.1x10-4). Knockdown of the transcription factor predicted to bind the enhancer region (NHLH1) in a human cell line (HEK293) expressing NHLH1 resulted in lower TSLP expression. In addition, we observed evidence of recent positive selection acting on the risk allele in populations of African descent. Our findings provide novel genetic leads to factors that influence mortality in patients with heart failure.
Journal: Plos Genetics, 2016 May DOI: 10.1371/journal.pgen.1006034 PMID: 27149122.0 PMCID: PMC4858216
157. Improved Identification and Analysis of Small Open Reading Frame Encoded Polypeptides.
Ma, Diedrich, Jungreis, Donaldson, Vaughan, Kellis, Yates, Saghatelian
Computational, genomic, and proteomic approaches have been used to discover nonannotated protein-coding small open reading frames (smORFs). Some novel smORFs have crucial biological roles in cells and organisms, which motivates the search for additional smORFs. Proteomic smORF discovery methods are advantageous because they detect smORF-encoded polypeptides (SEPs) to validate smORF translation and SEP stability. Because SEPs are shorter and less abundant than average proteins, SEP detection using proteomics faces unique challenges. Here, we optimize several steps in the SEP discovery workflow to improve SEP isolation and identification. These changes have led to the detection of several new human SEPs (novel human genes), improved confidence in the SEP assignments, and enabled quantification of SEPs under different cellular conditions. These improvements will allow faster detection and characterization of new SEPs and smORFs.
Journal: Analytical Chemistry, 2016 Apr 5 DOI: 10.1021/acs.analchem.6b00191 PMID: 27010111.0 PMCID: PMC4939623
156. Tissue-specific regulatory circuits reveal variable modular perturbations across complex diseases.
Marbach, Lamparter, Quon, Kellis, Kutalik, Bergmann
Mapping perturbed molecular circuits that underlie complex diseases remains a great challenge. We developed a comprehensive resource of 394 cell type- and tissue-specific gene regulatory networks for human, each specifying the genome-wide connectivity among transcription factors, enhancers, promoters and genes. Integration with 37 genome-wide association studies (GWASs) showed that disease-associated genetic variants--including variants that do not reach genome-wide significance--often perturb regulatory modules that are highly specific to disease-relevant cell types or tissues. Our resource opens the door to systematic analysis of regulatory programs across hundreds of human cell types and tissues (http://regulatorycircuits.org).
Journal: Nature Methods, 2016 Apr DOI: 10.1038/nmeth.3799 PMID: 26950747.0 PMCID: PMC4967716
155. Survey of variation in human transcription factors reveals prevalent DNA binding changes.
Barrera, Vedenko, Kurland, Rogers, Gisselbrecht, Rossin, Woodard, Mariani, Kock, Inukai, Siggers, Shokri, Gordan, Sahni, Cotsapas, Hao, Yi, Kellis, Daly, Vidal, Hill, Bulyk
Sequencing of exomes and genomes has revealed abundant genetic variation affecting the coding sequences of human transcription factors (TFs), but the consequences of such variation remain largely unexplored. We developed a computational, structure-based approach to evaluate TF variants for their impact on DNA binding activity and used universal protein-binding microarrays to assay sequence-specific DNA binding activity across 41 reference and 117 variant alleles found in individuals of diverse ancestries and families with Mendelian diseases. We found 77 variants in 28 genes that affect DNA binding affinity or specificity and identified thousands of rare alleles likely to alter the DNA binding activity of human sequence-specific TFs. Our results suggest that most individuals have unique repertoires of TF DNA binding activities, which may contribute to phenotypic variation.
Journal: Science, 2016 Mar 25 DOI: 10.1126/science.aad2257 PMID: 27013732.0 PMCID: PMC4825693
154. Corrigendum: Synchronized age-related gene expression changes across multiple tissues in human and the link to complex diseases.
Yang, Huang, Petralia, Long, Zhang, Argmann, Zhao, Mobbs, Schadt, Zhu, Tu
Aging is one of the most important biological processes and is a known risk factor for many age- related diseases in human. Studying age-related transcriptomic changes in tissues across the whole body can provide valuable information for a holistic understanding of this fundamental process. In this work, we catalogue age-related gene expression changes in nine tissues from nearly two hundred individuals collected by the Genotype-Tissue Expression (GTEx) project. In general, we find the aging gene expression signatures are very tissue specific. However, enrichment for some well- known aging components such as mitochondria biology is observed in many tissues. Different levels of cross-tissue synchronization of age-related gene expression changes are observed, and some essential tissues (e.g., heart and lung) show much stronger “co-aging” than other tissues based on a principal component analysis. The aging gene signatures and complex disease genes show a complex overlapping pattern and only in some cases, we see that they are significantly overlapped in the tissues affected by the corresponding diseases. In summary, our analyses provide novel insights to the co-regulation of age-related gene expression in multiple tissues; it also presents a tissue-specific view of the link between aging and age-related diseases.
Journal: Scientific Reports, 2016 Jan 21 DOI: 10.1038/srep19384 PMID: 26795431.0 PMCID: PMC4726320
153. FTO Obesity Variant and Adipocyte Browning in Humans.
Claussnitzer, Hui, Kellis
Correspondence
Journal: The New England Journal Of Medicine, 2016 Jan 14 DOI: 10.1056/NEJMc1513316 PMID: 26760096.0
152. HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease.
Ward, Kellis
More than 90% of common variants associated with complex traits do not affect proteins directly, but instead the circuits that control gene expression. This has increased the urgency of understanding the regulatory genome as a key component for translating genetic results into mechanistic insights and ultimately therapeutics. To address this challenge, we developed HaploReg (http://compbio.mit.edu/HaploReg) to aid the functional dissection of genome-wide association study (GWAS) results, the prediction of putative causal variants in haplotype blocks, the prediction of likely cell types of action, and the prediction of candidate target genes by systematic mining of comparative, epigenomic and regulatory annotations. Since first launching the website in 2011, we have greatly expanded HaploReg, increasing the number of chromatin state maps to 127 reference epigenomes from ENCODE 2012 and Roadmap Epigenomics, incorporating regulator binding data, expanding regulatory motif disruption annotations, and integrating expression quantitative trait locus (eQTL) variants and their tissue-specific target genes from GTEx, Geuvadis, and other recent studies. We present these updates as HaploReg v4, and illustrate a use case of HaploReg for attention deficit hyperactivity disorder (ADHD)-associated SNPs with putative brain regulatory mechanisms.
Journal: Nucleic Acids Research, 2016 Jan 4 DOI: 10.1093/nar/gkv1340 PMID: 26657631.0 PMCID: PMC4702929
151. Synchronized age-related gene expression changes across multiple tissues in human and the link to complex diseases.
Yang, Huang, Petralia, Long, Zhang, Argmann, Zhao, Mobbs, Schadt, Zhu, Tu
Aging is one of the most important biological processes and is a known risk factor for many age-related diseases in human. Studying age-related transcriptomic changes in tissues across the whole body can provide valuable information for a holistic understanding of this fundamental process. In this work, we catalogue age-related gene expression changes in nine tissues from nearly two hundred individuals collected by the Genotype-Tissue Expression (GTEx) project. In general, we find the aging gene expression signatures are very tissue specific. However, enrichment for some well-known aging components such as mitochondria biology is observed in many tissues. Different levels of cross-tissue synchronization of age-related gene expression changes are observed, and some essential tissues (e.g., heart and lung) show much stronger "co-aging" than other tissues based on a principal component analysis. The aging gene signatures and complex disease genes show a complex overlapping pattern and only in some cases, we see that they are significantly overlapped in the tissues affected by the corresponding diseases. In summary, our analyses provide novel insights to the co-regulation of age-related gene expression in multiple tissues; it also presents a tissue-specific view of the link between aging and age-related diseases.
Journal: Scientific Reports, 2015 Oct 19 DOI: 10.1038/srep15145 PMID: 26477495.0 PMCID: PMC4609956
150. A Novel Approach to High-Quality Postmortem Tissue Procurement: The GTEx Project.
Carithers, Ardlie, Barcus, Branton, Britton, Buia, Compton, DeLuca, Peter-Demchok, Gelfand, Guan, Korzeniewski, Lockhart, Rabiner, Rao, Robinson, Roche, Sawyer, Segre, Shive, Smith, Sobin, Undale, Valentino, Vaught, Young, Moore
The Genotype-Tissue Expression (GTEx) project, sponsored by the NIH Common Fund, was established to study the correlation between human genetic variation and tissue-specific gene expression in non-diseased individuals. A significant challenge was the collection of high-quality biospecimens for extensive genomic analyses. Here we describe how a successful infrastructure for biospecimen procurement was developed and implemented by multiple research partners to support the prospective collection, annotation, and distribution of blood, tissues, and cell lines for the GTEx project. Other research projects can follow this model and form beneficial partnerships with rapid autopsy and organ procurement organizations to collect high quality biospecimens and associated clinical data for genomic studies. Biospecimens, clinical and genomic data, and Standard Operating Procedures guiding biospecimen collection for the GTEx project are available to the research community.
Journal: Biopreservation And Biobanking, 2015 Oct DOI: 10.1089/bio.2015.0032 PMID: 26484571.0 PMCID: PMC4675181
149. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans.
Claussnitzer, Dankel, Kim, Quon, Meuleman, Haugen, Glunk, Sousa, Beaudry, Puviindran, Abdennur, Liu, Svensson, Hsu, Drucker, Mellgren, Hui, Hauner, Kellis
Genomewide association studies can be used to identify disease-relevant genomic regions, but interpretation of the data is challenging. The FTO region harbors the strongest genetic association with obesity, yet the mechanistic basis of this association remains elusive. METHODS: We examined epigenomic data, allelic activity, motif conservation, regulator expression, and gene coexpression patterns, with the aim of dissecting the regulatory circuitry and mechanistic basis of the association between the FTO region and obesity. We validated our predictions with the use of directed perturbations in samples from patients and from mice and with endogenous CRISPR-Cas9 genome editing in samples from patients. RESULTS: Our data indicate that the FTO allele associated with obesity represses mitochondrial thermogenesis in adipocyte precursor cells in a tissue-autonomous manner. The rs1421085 T-to-C single-nucleotide variant disrupts a conserved motif for the ARID5B repressor, which leads to derepression of a potent preadipocyte enhancer and a doubling of IRX3 and IRX5 expression during early adipocyte differentiation. This results in a cell-autonomous developmental shift from energy-dissipating beige (brite) adipocytes to energy-storing white adipocytes, with a reduction in mitochondrial thermogenesis by a factor of 5, as well as an increase in lipid storage. Inhibition of Irx3 in adipose tissue in mice reduced body weight and increased energy dissipation without a change in physical activity or appetite. Knockdown of IRX3 or IRX5 in primary adipocytes from participants with the risk allele restored thermogenesis, increasing it by a factor of 7, and overexpression of these genes had the opposite effect in adipocytes from nonrisk-allele carriers. Repair of the ARID5B motif by CRISPR-Cas9 editing of rs1421085 in primary adipocytes from a patient with the risk allele restored IRX3 and IRX5 repression, activated browning expression programs, and restored thermogenesis, increasing it by a factor of 7. CONCLUSIONS: Our results point to a pathway for adipocyte thermogenesis regulation involving ARID5B, rs1421085, IRX3, and IRX5, which, when manipulated, had pronounced pro-obesity and anti-obesity effects. (Funded by the German Research Center for Environmental Health and others.).
Journal: The New England Journal Of Medicine, 2015 Sep 3 DOI: 10.1056/NEJMoa1502214 PMID: 26287746.0 PMCID: PMC4959911
148. PRC2 Is Required to Maintain Expression of the Maternal Gtl2-Rian-Mirg Locus by Preventing De Novo DNA Methylation in Mouse Embryonic Stem Cells.
Das, Hendrix, Apostolou, Buchner, Canver, Beyaz, Ljuboja, Kuintzle, Kim, Karnik, Shao, Xie, Xu, De, Zhang, Choe, Jun, Shen, Gregory, Daley, Meissner, Kellis, Hochedlinger, Kim, Orkin
Polycomb Repressive Complex 2 (PRC2) function and DNA methylation (DNAme) are typically correlated with gene repression. Here, we show that PRC2 is required to maintain expression of maternal microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) from the Gtl2-Rian-Mirg locus, which is essential for full pluripotency of iPSCs. In the absence of PRC2, the entire locus becomes transcriptionally repressed due to gain of DNAme at the intergenic differentially methylated regions (IG-DMRs). Furthermore, we demonstrate that the IG-DMR serves as an enhancer of the maternal Gtl2-Rian-Mirg locus. Further analysis reveals that PRC2 interacts physically with Dnmt3 methyltransferases and reduces recruitment to and subsequent DNAme at the IG-DMR, thereby allowing for proper expression of the maternal Gtl2-Rian-Mirg locus. Our observations are consistent with a mechanism through which PRC2 counteracts the action of Dnmt3 methyltransferases at an imprinted locus required for full pluripotency.
Journal: Cell Reports, 2015 Sep 1 DOI: 10.1016/j.celrep.2015.07.053 PMID: 26299972.0 PMCID: PMC5384103
147. Systematic chromatin state comparison of epigenomes associated with diverse properties including sex and tissue type.
Yen, Kellis
Epigenomic data sets provide critical information about the dynamic role of chromatin states in gene regulation, but a key question of how chromatin state segmentations vary under different conditions across the genome has remained unaddressed. Here we present ChromDiff, a group-wise chromatin state comparison method that generates an information-theoretic representation of epigenomes and corrects for external covariate factors to better isolate relevant chromatin state changes. By applying ChromDiff to the 127 epigenomes from the Roadmap Epigenomics and ENCODE projects, we provide novel group-wise comparative analyses across sex, tissue type, state and developmental age. Remarkably, we find that distinct sets of epigenomic features are maximally discriminative for different group-wise comparisons, in each case revealing distinct enriched pathways, many of which do not show gene expression differences. Our methodology should be broadly applicable for epigenomic comparisons and provides a powerful new tool for studying chromatin state differences at the genome scale.
Journal: Nature Communications, 2015 Aug 18 DOI: 10.1038/ncomms8973 PMID: 26282110.0 PMCID: PMC4557131
146. Deep learning for regulatory genomics.
Park, Kellis
Journal: Nature Biotechnology, 2015 Aug DOI: 10.1038/nbt.3313 PMID: 26252139.0
145. Activity-Induced DNA Breaks Govern the Expression of Neuronal Early-Response Genes.
Madabhushi, Gao, Pfenning, Pan, Yamakawa, Seo, Rueda, Phan, Yamakawa, Pao, Stott, Gjoneska, Nott, Cho, Kellis, Tsai
Neuronal activity causes the rapid expression of immediate early genes that are crucial for experience-driven changes to synapses, learning, and memory. Here, using both molecular and genome-wide next-generation sequencing methods, we report that neuronal activity stimulation triggers the formation of DNA double strand breaks (DSBs) in the promoters of a subset of early-response genes, including Fos, Npas4, and Egr1. Generation of targeted DNA DSBs within Fos and Npas4 promoters is sufficient to induce their expression even in the absence of an external stimulus. Activity-dependent DSB formation is likely mediated by the type II topoisomerase, Topoisomerase IIbeta (Topo IIbeta), and knockdown of Topo IIbeta attenuates both DSB formation and early-response gene expression following neuronal stimulation. Our results suggest that DSB formation is a physiological event that rapidly resolves topological constraints to early-response gene expression in neurons.
Journal: Cell, 2015 Jun 18 DOI: 10.1016/j.cell.2015.05.032 PMID: 26052046.0 PMCID: PMC4886855
144. Context influences on TALE-DNA binding revealed by quantitative profiling.
Rogers, Barrera, Reyon, Sander, Kellis, Joung, Bulyk
Transcription activator-like effector (TALE) proteins recognize DNA using a seemingly simple DNA-binding code, which makes them attractive for use in genome engineering technologies that require precise targeting. Although this code is used successfully to design TALEs to target specific sequences, off-target binding has been observed and is difficult to predict. Here we explore TALE-DNA interactions comprehensively by quantitatively assaying the DNA-binding specificities of 21 representative TALEs to approximately 5,000-20,000 unique DNA sequences per protein using custom-designed protein-binding microarrays (PBMs). We find that protein context features exert significant influences on binding. Thus, the canonical recognition code does not fully capture the complexity of TALE-DNA binding. We used the PBM data to develop a computational model, Specificity Inference For TAL-Effector Design (SIFTED), to predict the DNA-binding specificity of any TALE. We provide SIFTED as a publicly available web tool that predicts potential genomic off-target sites for improved TALE design.
Journal: Nature Communications, 2015 Jun 11 DOI: 10.1038/ncomms8440 PMID: 26067805.0 PMCID: PMC4467457
143. Alzheimer's loci: epigenetic associations and interaction with genetic factors.
Chibnik, Yu, Eaton, Srivastava, Schneider, Kellis, Bennett, De
We explore the role of DNA methylation in Alzheimer's disease (AD). To elucidate where DNA methylation falls along the causal pathway linking risk factors to disease, we examine causal models to assess its role in the pathology of AD. METHODS: DNA methylation profiles were generated in 740 brain samples using the Illumina HumanMet450K beadset. We focused our analysis on CpG sites from 11 AD susceptibility gene regions. The primary outcome was a quantitative measure of neuritic amyloid plaque (NP), a key early element of AD pathology. We tested four causal models: (1) independent associations, (2) CpG mediating the association of a variant, (3) reverse causality, and (4) genetic variant by CpG interaction. RESULTS: Six genes regions (17 CpGs) showed evidence of CpG associations with NP, independent of genetic variation - BIN1 (5), CLU (5), MS4A6A (3), ABCA7 (2), CD2AP (1), and APOE (1). Together they explained 16.8% of the variability in NP. An interaction effect was seen in the CR1 region for two CpGs, cg10021878 (P = 0.01) and cg05922028 (P = 0.001), in relation to NP. In both cases, subjects with the risk allele rs6656401(AT) (/) (AA) display more methylation being associated with more NP burden, whereas subjects with the rs6656401(TT) protective genotype have an inverse association with more methylation being associated with less NP. INTERPRETATION: These observations suggest that, within known AD susceptibility loci, methylation is related to pathologic processes of AD and may play a largely independent role by influencing gene expression in AD susceptibility loci.
Journal: Annals Of Clinical And Translational Neurology, 2015 Jun DOI: 10.1002/acn3.201 PMID: 26125039.0 PMCID: PMC4479524
142. Human genomics. Effect of predicted protein-truncating genetic variants on the human transcriptome.
Rivas, Pirinen, Conrad, Lek, Tsang, Karczewski, Maller, Kukurba, DeLuca, Fromer, Ferreira, Smith, Zhang, Zhao, Banks, Poplin, Ruderfer, Purcell, Tukiainen, Minikel, Stenson, Cooper, Huang, Sullivan, Nedzel, Bustamante, Li, Daly, Guigo, Donnelly, Ardlie, Sammeth, Dermitzakis, McCarthy, Montgomery, Lappalainen, MacArthur
Accurate prediction of the functional effect of genetic variation is critical for clinical genome interpretation. We systematically characterized the transcriptome effects of protein-truncating variants, a class of variants expected to have profound effects on gene function, using data from the Genotype-Tissue Expression (GTEx) and Geuvadis projects. We quantitated tissue-specific and positional effects on nonsense-mediated transcript decay and present an improved predictive model for this decay. We directly measured the effect of variants both proximal and distal to splice junctions. Furthermore, we found that robustness to heterozygous gene inactivation is not due to dosage compensation. Our results illustrate the value of transcriptome data in the functional interpretation of genetic variants.
Journal: Science, 2015 May 8 DOI: 10.1126/science.1261877 PMID: 25954003.0 PMCID: PMC4537935
141. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans.
The GTEx Consortium
Understanding the functional consequences of genetic variation, and how it affects complex human disease and quantitative traits, remains a critical challenge for biomedicine. We present an analysis of RNA sequencing data from 1641 samples across 43 tissues from 175 individuals, generated as part of the pilot phase of the Genotype-Tissue Expression (GTEx) project. We describe the landscape of gene expression across tissues, catalog thousands of tissue-specific and shared regulatory expression quantitative trait loci (eQTL) variants, describe complex network relationships, and identify signals from genome-wide association studies explained by eQTLs. These findings provide a systematic understanding of the cellular and biological consequences of human genetic variation and of the heterogeneity of such effects among a diverse set of human tissues.
Journal: Science, 2015 May 8 DOI: 10.1126/science.1262110 PMID: 25954001.0 PMCID: PMC4547484
140. Sharing and Specificity of Co-expression Networks across 35 Human Tissues.
Pierson, Koller, Battle, Mostafavi, Ardlie, Getz, Wright, Kellis, Volpi, Dermitzakis
To understand the regulation of tissue-specific gene expression, the GTEx Consortium generated RNA-seq expression data for more than thirty distinct human tissues. This data provides an opportunity for deriving shared and tissue specific gene regulatory networks on the basis of co-expression between genes. However, a small number of samples are available for a majority of the tissues, and therefore statistical inference of networks in this setting is highly underpowered. To address this problem, we infer tissue-specific gene co-expression networks for 35 tissues in the GTEx dataset using a novel algorithm, GNAT, that uses a hierarchy of tissues to share data between related tissues. We show that this transfer learning approach increases the accuracy with which networks are learned. Analysis of these networks reveals that tissue-specific transcription factors are hubs that preferentially connect to genes with tissue specific functions. Additionally, we observe that genes with tissue-specific functions lie at the peripheries of our networks. We identify numerous modules enriched for Gene Ontology functions, and show that modules conserved across tissues are especially likely to have functions common to all tissues, while modules that are upregulated in a particular tissue are often instrumental to tissue-specific function. Finally, we provide a web tool, available at mostafavilab.stat.ubc.ca/GNAT, which allows exploration of gene function and regulation in a tissue-specific manner.
Journal: Plos Computational Biology, 2015 May DOI: 10.1371/journal.pcbi.1004220 PMID: 25970446.0 PMCID: PMC4430528
139. Improved gene tree error correction in the presence of horizontal gene transfer.
Bansal, Wu, Alm, Kellis
The accurate inference of gene trees is a necessary step in many evolutionary studies. Although the problem of accurate gene tree inference has received considerable attention, most existing methods are only applicable to gene families unaffected by horizontal gene transfer. As a result, the accurate inference of gene trees affected by horizontal gene transfer remains a largely unaddressed problem. RESULTS: In this study, we introduce a new and highly effective method for gene tree error correction in the presence of horizontal gene transfer. Our method efficiently models horizontal gene transfers, gene duplications and losses, and uses a statistical hypothesis testing framework [Shimodaira-Hasegawa (SH) test] to balance sequence likelihood with topological information from a known species tree. Using a thorough simulation study, we show that existing phylogenetic methods yield inaccurate gene trees when applied to horizontally transferred gene families and that our method dramatically improves gene tree accuracy. We apply our method to a dataset of 11 cyanobacterial species and demonstrate the large impact of gene tree accuracy on downstream evolutionary analyses. AVAILABILITY AND IMPLEMENTATION: An implementation of our method is available at http://compbio.mit.edu/treefix-dtl/ CONTACT: : mukul@engr.uconn.edu or manoli@mit.edu SUPPLEMENTARY INFORMATION: Supplementary data are available at Bioinformatics online.
Journal: Bioinformatics, 2015 Apr 15 DOI: 10.1093/bioinformatics/btu806 PMID: 25481006.0 PMCID: PMC4393519
138. Large-scale imputation of epigenomic datasets for systematic annotation of diverse human tissues.
Ernst, Kellis
With hundreds of epigenomic maps, the opportunity arises to exploit the correlated nature of epigenetic signals, across both marks and samples, for large-scale prediction of additional datasets. Here, we undertake epigenome imputation by leveraging such correlations through an ensemble of regression trees. We impute 4,315 high-resolution signal maps, of which 26% are also experimentally observed. Imputed signal tracks show overall similarity to observed signals and surpass experimental datasets in consistency, recovery of gene annotations and enrichment for disease-associated variants. We use the imputed data to detect low-quality experimental datasets, to find genomic sites with unexpected epigenomic signals, to define high-priority marks for new experiments and to delineate chromatin states in 127 reference epigenomes spanning diverse tissues and cell types. Our imputed datasets provide the most comprehensive human regulatory region annotation to date, and our approach and the ChromImpute software constitute a useful complement to large-scale experimental mapping of epigenomic information.
Journal: Nature Biotechnology, 2015 Apr DOI: 10.1038/nbt.3157 PMID: 25690853.0 PMCID: PMC4512306
137. Corrigendum: Network deconvolution as a general method to distinguish direct dependencies in networks.
Feizi, Marbach, Medard, Kellis
Recognizing direct relationships between variables connected in a network is a pervasive problem in biological, social and information sciences as correlation-based networks contain numerous indirect relationships. Here we present a general method for inferring direct effects from an observed correlation matrix containing both direct and indirect effects. We formulate the problem as the inverse of network convolution, and introduce an algorithm that removes the combined effect of all indirect paths of arbitrary length in a closed-form solution by exploiting eigen-decomposition and infinite-series sums. We demonstrate the effectiveness of our approach in several network applications: distinguishing direct targets in gene expression regulatory networks; recognizing directly interacting amino-acid residues for protein structure prediction from sequence alignments; and distinguishing strong collaborations in co-authorship social networks using connectivity information alone. In addition to its theoretical impact as a foundational graph theoretic tool, our results suggest network deconvolution is widely applicable for computing direct dependencies in network science across diverse disciplines.
Journal: Nature Biotechnology, 2015 Apr DOI: 10.1038/nbt0415-424 PMID: 25850062.0
136. Preface: RECOMB/ISCB systems biology, regulatory genomics, and DREAM 2014 Special Issue.
Califano, Kellis, Stolovitzky
Journal: Journal Of Computational Biology, 2015 Apr DOI: 10.1089/cmb.2015.020P PMID: 25844665.0
135. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers.
Onengut-Gumuscu, Chen, Burren, Cooper, Quinlan, Mychaleckyj, Farber, Bonnie, Szpak, Schofield, Achuthan, Guo, Fortune, Stevens, Walker, Ward, Kundaje, Kellis, Daly, Barrett, Cooper, Deloukas, Todd, Wallace, Concannon, Rich
Genetic studies of type 1 diabetes (T1D) have identified 50 susceptibility regions, finding major pathways contributing to risk, with some loci shared across immune disorders. To make genetic comparisons across autoimmune disorders as informative as possible, a dense genotyping array, the Immunochip, was developed, from which we identified four new T1D-associated regions (P < 5 x 10(-8)). A comparative analysis with 15 immune diseases showed that T1D is more similar genetically to other autoantibody-positive diseases, significantly most similar to juvenile idiopathic arthritis and significantly least similar to ulcerative colitis, and provided support for three additional new T1D risk loci. Using a Bayesian approach, we defined credible sets for the T1D-associated SNPs. The associated SNPs localized to enhancer sequences active in thymus, T and B cells, and CD34(+) stem cells. Enhancer-promoter interactions can now be analyzed in these cell types to identify which particular genes and regulatory sequences are causal.
Journal: Nature Genetics, 2015 Apr DOI: 10.1038/ng.3245 PMID: 25751624.0 PMCID: PMC4380767
134. BRCA1 recruitment to transcriptional pause sites is required for R-loop-driven DNA damage repair.
Hatchi, Skourti-Stathaki, Ventz, Pinello, Yen, Kamieniarz-Gdula, Dimitrov, Pathania, McKinney, Eaton, Kellis, Hill, Parmigiani, Proudfoot, Livingston
The mechanisms contributing to transcription-associated genomic instability are both complex and incompletely understood. Although R-loops are normal transcriptional intermediates, they are also associated with genomic instability. Here, we show that BRCA1 is recruited to R-loops that form normally over a subset of transcription termination regions. There it mediates the recruitment of a specific, physiological binding partner, senataxin (SETX). Disruption of this complex led to R-loop-driven DNA damage at those loci as reflected by adjacent gamma-H2AX accumulation and ssDNA breaks within the untranscribed strand of relevant R-loop structures. Genome-wide analysis revealed widespread BRCA1 binding enrichment at R-loop-rich termination regions (TRs) of actively transcribed genes. Strikingly, within some of these genes in BRCA1 null breast tumors, there are specific insertion/deletion mutations located close to R-loop-mediated BRCA1 binding sites within TRs. Thus, BRCA1/SETX complexes support a DNA repair mechanism that addresses R-loop-based DNA damage at transcriptional pause sites.
Journal: Molecular Cell, 2015 Feb 19 DOI: 10.1016/j.molcel.2015.01.011 PMID: 25699710.0 PMCID: PMC4351672
133. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer's disease.
Gjoneska, Pfenning, Mathys, Quon, Kundaje, Tsai, Kellis
Alzheimer's disease (AD) is a severe age-related neurodegenerative disorder characterized by accumulation of amyloid-beta plaques and neurofibrillary tangles, synaptic and neuronal loss, and cognitive decline. Several genes have been implicated in AD, but chromatin state alterations during neurodegeneration remain uncharacterized. Here we profile transcriptional and chromatin state dynamics across early and late pathology in the hippocampus of an inducible mouse model of AD-like neurodegeneration. We find a coordinated downregulation of synaptic plasticity genes and regulatory regions, and upregulation of immune response genes and regulatory regions, which are targeted by factors that belong to the ETS family of transcriptional regulators, including PU.1. Human regions orthologous to increasing-level enhancers show immune-cell-specific enhancer signatures as well as immune cell expression quantitative trait loci, while decreasing-level enhancer orthologues show fetal-brain-specific enhancer activity. Notably, AD-associated genetic variants are specifically enriched in increasing-level enhancer orthologues, implicating immune processes in AD predisposition. Indeed, increasing enhancers overlap known AD loci lacking protein-altering variants, and implicate additional loci that do not reach genome-wide significance. Our results reveal new insights into the mechanisms of neurodegeneration and establish the mouse as a useful model for functional studies of AD regulatory regions.
Journal: Nature, 2015 Feb 19 DOI: 10.1038/nature14252 PMID: 25693568.0 PMCID: PMC4530583
132. Integrative analysis of 111 reference human epigenomes.
Kundaje, Meuleman, Ernst, Bilenky, Yen, Heravi-Moussavi, Kheradpour, Zhang, Wang, Ziller, Amin, Whitaker, Schultz, Ward, Sarkar, Quon, Sandstrom, Eaton, Wu, Pfenning, Wang, Claussnitzer, Liu, Coarfa, Harris, Shoresh, Epstein, Gjoneska, Leung, Xie, Hawkins, Lister, Hong, Gascard, Mungall, Moore, Chuah, Tam, Canfield, Hansen, Kaul, Sabo, Bansal, Carles, Dixon, Farh, Feizi, Karlic, Kim, Kulkarni, Li, Lowdon, Elliott, Mercer, Neph, Onuchic, Polak, Rajagopal, Ray, Sallari, Siebenthall, Sinnott-Armstrong, Stevens, Thurman, Wu, Zhang, Zhou, Beaudet, Boyer, De, Farnham, Fisher, Haussler, Jones, Li, Marra, McManus, Sunyaev, Thomson, Tlsty, Tsai, Wang, Waterland, Zhang, Chadwick, Bernstein, Costello, Ecker, Hirst, Meissner, Milosavljevic, Ren, Stamatoyannopoulos, Wang, Kellis
The reference human genome sequence set the stage for studies of genetic variation and its association with human disease, but epigenomic studies lack a similar reference. To address this need, the NIH Roadmap Epigenomics Consortium generated the largest collection so far of human epigenomes for primary cells and tissues. Here we describe the integrative analysis of 111 reference human epigenomes generated as part of the programme, profiled for histone modification patterns, DNA accessibility, DNA methylation and RNA expression. We establish global maps of regulatory elements, define regulatory modules of coordinated activity, and their likely activators and repressors. We show that disease- and trait-associated genetic variants are enriched in tissue-specific epigenomic marks, revealing biologically relevant cell types for diverse human traits, and providing a resource for interpreting the molecular basis of human disease. Our results demonstrate the central role of epigenomic information for understanding gene regulation, cellular differentiation and human disease.
Journal: Nature, 2015 Feb 19 DOI: 10.1038/nature14248 PMID: 25693563.0 PMCID: PMC4530010
131. Intermediate DNA methylation is a conserved signature of genome regulation.
Elliott, Hong, Xing, Zhou, Li, Coarfa, Bell, Maire, Ligon, Sigaroudinia, Gascard, Tlsty, Harris, Schalkwyk, Bilenky, Mill, Farnham, Kellis, Marra, Milosavljevic, Hirst, Stormo, Wang, Costello
The role of intermediate methylation states in DNA is unclear. Here, to comprehensively identify regions of intermediate methylation and their quantitative relationship with gene activity, we apply integrative and comparative epigenomics to 25 human primary cell and tissue samples. We report 18,452 intermediate methylation regions located near 36% of genes and enriched at enhancers, exons and DNase I hypersensitivity sites. Intermediate methylation regions average 57% methylation, are predominantly allele-independent and are conserved across individuals and between mouse and human, suggesting a conserved function. These regions have an intermediate level of active chromatin marks and their associated genes have intermediate transcriptional activity. Exonic intermediate methylation correlates with exon inclusion at a level between that of fully methylated and unmethylated exons, highlighting gene context-dependent functions. We conclude that intermediate DNA methylation is a conserved signature of gene regulation and exon usage.
Journal: Nature Communications, 2015 Feb 18 DOI: 10.1038/ncomms7363 PMID: 25691127.0 PMCID: PMC4333717
130. FRESCo: finding regions of excess synonymous constraint in diverse viruses.
Sealfon, Lin, Jungreis, Wolf, Kellis, Sabeti
The increasing availability of sequence data for many viruses provides power to detect regions under unusual evolutionary constraint at a high resolution. One approach leverages the synonymous substitution rate as a signature to pinpoint genic regions encoding overlapping or embedded functional elements. Protein-coding regions in viral genomes often contain overlapping RNA structural elements, reading frames, regulatory elements, microRNAs, and packaging signals. Synonymous substitutions in these regions would be selectively disfavored and thus these regions are characterized by excess synonymous constraint. Codon choice can also modulate transcriptional efficiency, translational accuracy, and protein folding. RESULTS: We developed a phylogenetic codon model-based framework, FRESCo, designed to find regions of excess synonymous constraint in short, deep alignments, such as individual viral genes across many sequenced isolates. We demonstrated the high specificity of our approach on simulated data and applied our framework to the protein-coding regions of approximately 30 distinct species of viruses with diverse genome architectures. CONCLUSIONS: FRESCo recovers known multifunctional regions in well-characterized viruses such as hepatitis B virus, poliovirus, and West Nile virus, often at a single-codon resolution, and predicts many novel functional elements overlapping viral genes, including in Lassa and Ebola viruses. In a number of viruses, the synonymously constrained regions that we identified also display conserved, stable predicted RNA structures, including putative novel elements in multiple viral species.
Journal: Genome Biology, 2015 Feb 17 DOI: 10.1186/s13059-015-0603-7 PMID: 25853568.0 PMCID: PMC4376164
129. Mosquito genomics. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes.
Neafsey, Waterhouse, Abai, Aganezov, Alekseyev, Allen, Amon, Arca, Arensburger, Artemov, Assour, Basseri, Berlin, Birren, Blandin, Brockman, Burkot, Burt, Chan, Chauve, Chiu, Christensen, Costantini, Davidson, Deligianni, Dottorini, Dritsou, Gabriel, Guelbeogo, Hall, Han, Hlaing, Hughes, Jenkins, Jiang, Jungreis, Kakani, Kamali, Kemppainen, Kennedy, Kirmitzoglou, Koekemoer, Laban, Langridge, Lawniczak, Lirakis, Lobo, Lowy, MacCallum, Mao, Maslen, Mbogo, McCarthy, Michel, Mitchell, Moore, Murphy, Naumenko, Nolan, Novoa, O'Loughlin, Oringanje, Oshaghi, Pakpour, Papathanos, Peery, Povelones, Prakash, Price, Rajaraman, Reimer, Rinker, Rokas, Russell, Sagnon, Sharakhova, Shea, Simao, Simard, Slotman, Somboon, Stegniy, Struchiner, Thomas, Tojo, Topalis, Tubio, Unger, Vontas, Walton, Wilding, Willis, Wu, Yan, Zdobnov, Zhou, Catteruccia, Christophides, Collins, Cornman, Crisanti, Donnelly, Emrich, Fontaine, Gelbart, Hahn, Hansen, Howell, Kafatos, Kellis, Lawson, Louis, Luckhart, Muskavitch, Ribeiro, Riehle, Sharakhov, Tu, Zwiebel, Besansky
Variation in vectorial capacity for human malaria among Anopheles mosquito species is determined by many factors, including behavior, immunity, and life history. To investigate the genomic basis of vectorial capacity and explore new avenues for vector control, we sequenced the genomes of 16 anopheline mosquito species from diverse locations spanning ~100 million years of evolution. Comparative analyses show faster rates of gene gain and loss, elevated gene shuffling on the X chromosome, and more intron losses, relative to Drosophila. Some determinants of vectorial capacity, such as chemosensory genes, do not show elevated turnover but instead diversify through protein-sequence changes. This dynamism of anopheline genes and genomes may contribute to their flexible capacity to take advantage of new ecological niches, including adapting to humans as primary hosts.
Journal: Science, 2015 Jan 2 DOI: 10.1126/science.1258522 PMID: 25554792.0 PMCID: PMC4380271
128. Core and region-enriched networks of behaviorally regulated genes and the singing genome.
Whitney, Pfenning, Howard, Blatti, Liu, Ward, Wang, Audet, Kellis, Mukherjee, Sinha, Hartemink, West, Jarvis
Songbirds represent an important model organism for elucidating molecular mechanisms that link genes with complex behaviors, in part because they have discrete vocal learning circuits that have parallels with those that mediate human speech. We found that ~10% of the genes in the avian genome were regulated by singing, and we found a striking regional diversity of both basal and singing-induced programs in the four key song nuclei of the zebra finch, a vocal learning songbird. The region-enriched patterns were a result of distinct combinations of region-enriched transcription factors (TFs), their binding motifs, and presinging acetylation of histone 3 at lysine 27 (H3K27ac) enhancer activity in the regulatory regions of the associated genes. RNA interference manipulations validated the role of the calcium-response transcription factor (CaRF) in regulating genes preferentially expressed in specific song nuclei in response to singing. Thus, differential combinatorial binding of a small group of activity-regulated TFs and predefined epigenetic enhancer activity influences the anatomical diversity of behaviorally regulated gene networks.
Journal: Science, 2014 Dec 12 DOI: 10.1126/science.1256780 PMID: 25504732.0 PMCID: PMC4359888
127. Principles of regulatory information conservation between mouse and human.
Cheng, Ma, Kim, Wu, Cayting, Boyle, Sundaram, Xing, Dogan, Li, Euskirchen, Lin, Lin, Visel, Kawli, Yang, Patacsil, Keller, Giardine, Kundaje, Wang, Pennacchio, Weng, Hardison, Snyder
To broaden our understanding of the evolution of gene regulation mechanisms, we generated occupancy profiles for 34 orthologous transcription factors (TFs) in human-mouse erythroid progenitor, lymphoblast and embryonic stem-cell lines. By combining the genome-wide transcription factor occupancy repertoires, associated epigenetic signals, and co-association patterns, here we deduce several evolutionary principles of gene regulatory features operating since the mouse and human lineages diverged. The genomic distribution profiles, primary binding motifs, chromatin states, and DNA methylation preferences are well conserved for TF-occupied sequences. However, the extent to which orthologous DNA segments are bound by orthologous TFs varies both among TFs and with genomic location: binding at promoters is more highly conserved than binding at distal elements. Notably, occupancy-conserved TF-occupied sequences tend to be pleiotropic; they function in several tissues and also co-associate with many TFs. Single nucleotide variants at sites with potential regulatory functions are enriched in occupancy-conserved TF-occupied sequences.
Journal: Nature, 2014 Nov 20 DOI: 10.1038/nature13985 PMID: 25409826.0 PMCID: PMC4343047
126. A comparative encyclopedia of DNA elements in the mouse genome.
Yue, Cheng, Breschi, Vierstra, Wu, Ryba, Sandstrom, Ma, Davis, Pope, Shen, Pervouchine, Djebali, Thurman, Kaul, Rynes, Kirilusha, Marinov, Williams, Trout, Amrhein, Fisher-Aylor, Antoshechkin, DeSalvo, See, Fastuca, Drenkow, Zaleski, Dobin, Prieto, Lagarde, Bussotti, Tanzer, Denas, Li, Bender, Zhang, Byron, Groudine, McCleary, Pham, Ye, Kuan, Edsall, Wu, Rasmussen, Bansal, Kellis, Keller, Morrissey, Mishra, Jain, Dogan, Harris, Cayting, Kawli, Boyle, Euskirchen, Kundaje, Lin, Lin, Jansen, Malladi, Cline, Erickson, Kirkup, Learned, Sloan, Rosenbloom, Lacerda, Beal, Pignatelli, Flicek, Lian, Kahveci, Lee, Kent, Ramalho, Herrero, Notredame, Johnson, Vong, Lee, Bates, Neri, Diegel, Canfield, Sabo, Wilken, Reh, Giste, Shafer, Kutyavin, Haugen, Dunn, Reynolds, Neph, Humbert, Hansen, De, Selleri, Rudensky, Josefowicz, Samstein, Eichler, Orkin, Levasseur, Papayannopoulou, Chang, Skoultchi, Gosh, Disteche, Treuting, Wang, Weiss, Blobel, Cao, Zhong, Wang, Good, Lowdon, Adams, Zhou, Pazin, Feingold, Wold, Taylor, Mortazavi, Weissman, Stamatoyannopoulos, Snyder, Guigo, Gingeras, Gilbert, Hardison, Beer, Ren
The laboratory mouse shares the majority of its protein-coding genes with humans, making it the premier model organism in biomedical research, yet the two mammals differ in significant ways. To gain greater insights into both shared and species-specific transcriptional and cellular regulatory programs in the mouse, the Mouse ENCODE Consortium has mapped transcription, DNase I hypersensitivity, transcription factor binding, chromatin modifications and replication domains throughout the mouse genome in diverse cell and tissue types. By comparing with the human genome, we not only confirm substantial conservation in the newly annotated potential functional sequences, but also find a large degree of divergence of sequences involved in transcriptional regulation, chromatin state and higher order chromatin organization. Our results illuminate the wide range of evolutionary forces acting on genes and their regulatory regions, and provide a general resource for research into mammalian biology and mechanisms of human diseases.
Journal: Nature, 2014 Nov 20 DOI: 10.1038/nature13992 PMID: 25409824.0 PMCID: PMC4266106
125. CHD8 regulates neurodevelopmental pathways associated with autism spectrum disorder in neural progenitors.
Sugathan, Biagioli, Golzio, Erdin, Blumenthal, Manavalan, Ragavendran, Brand, Lucente, Miles, Sheridan, Stortchevoi, Kellis, Haggarty, Katsanis, Gusella, Talkowski
Truncating mutations of chromodomain helicase DNA-binding protein 8 (CHD8), and of many other genes with diverse functions, are strong-effect risk factors for autism spectrum disorder (ASD), suggesting multiple mechanisms of pathogenesis. We explored the transcriptional networks that CHD8 regulates in neural progenitor cells (NPCs) by reducing its expression and then integrating transcriptome sequencing (RNA sequencing) with genome-wide CHD8 binding (ChIP sequencing). Suppressing CHD8 to levels comparable with the loss of a single allele caused altered expression of 1,756 genes, 64.9% of which were up-regulated. CHD8 showed widespread binding to chromatin, with 7,324 replicated sites that marked 5,658 genes. Integration of these data suggests that a limited array of direct regulatory effects of CHD8 produced a much larger network of secondary expression changes. Genes indirectly down-regulated (i.e., without CHD8-binding sites) reflect pathways involved in brain development, including synapse formation, neuron differentiation, cell adhesion, and axon guidance, whereas CHD8-bound genes are strongly associated with chromatin modification and transcriptional regulation. Genes associated with ASD were strongly enriched among indirectly down-regulated loci (P < 10(-8)) and CHD8-bound genes (P = 0.0043), which align with previously identified coexpression modules during fetal development. We also find an intriguing enrichment of cancer-related gene sets among CHD8-bound genes (P < 10(-10)). In vivo suppression of chd8 in zebrafish produced macrocephaly comparable to that of humans with inactivating mutations. These data indicate that heterozygous disruption of CHD8 precipitates a network of gene-expression changes involved in neurodevelopmental pathways in which many ASD-associated genes may converge on shared mechanisms of pathogenesis.
Journal: Proceedings Of The National Academy Of Sciences Of The United States Of America, 2014 Oct 21 DOI: 10.1073/pnas.1405266111 PMID: 25294932.0 PMCID: PMC4210312
124. The NF-kappaB genomic landscape in lymphoblastoid B cells.
Zhao, Barrera, Ersing, Willox, Schmidt, Greenfeld, Zhou, Mollo, Shi, Takasaki, Jiang, Cahir-McFarland, Kellis, Bulyk, Kieff, Gewurz
The nuclear factor kappaB (NF-kappaBeta) subunits RelA, RelB, cRel, p50, and p52 are each critical for B cell development and function. To systematically characterize their responses to canonical and noncanonical NF-kappaB pathway activity, we performed chromatin immunoprecipitation followed by high-throughput DNA sequencing (ChIP-seq) analysis in lymphoblastoid B cell lines (LCLs). We found a complex NF-kappaB-binding landscape, which did not readily reflect the two NF-kappaB pathway paradigms. Instead, 10 subunit-binding patterns were observed at promoters and 11 at enhancers. Nearly one-third of NF-kappaB-binding sites lacked kappaB motifs and were instead enriched for alternative motifs. The oncogenic forkhead box protein FOXM1 co-occupied nearly half of NF-kappaB-binding sites and was identified in protein complexes with NF-kappaB on DNA. FOXM1 knockdown decreased NF-kappaB target gene expression and ultimately induced apoptosis, highlighting FOXM1 as a synthetic lethal target in B cell malignancy. These studies provide a resource for understanding mechanisms that underlie NF-kappaB nuclear activity and highlight opportunities for selective NF-kappaB blockade.
Journal: Cell Reports, 2014 Sep 11 DOI: 10.1016/j.celrep.2014.07.037 PMID: 25159142.0 PMCID: PMC4163118
123. Alzheimer's disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci.
De, Srivastava, Lunnon, Burgess, Schalkwyk, Yu, Eaton, Keenan, Ernst, McCabe, Tang, Raj, Replogle, Brodeur, Gabriel, Chai, Younkin, Younkin, Zou, Szyf, Epstein, Schneider, Bernstein, Meissner, Ertekin-Taner, Chibnik, Kellis, Mill, Bennett
We used a collection of 708 prospectively collected autopsied brains to assess the methylation state of the brain's DNA in relation to Alzheimer's disease (AD). We found that the level of methylation at 71 of the 415,848 interrogated CpGs was significantly associated with the burden of AD pathology, including CpGs in the ABCA7 and BIN1 regions, which harbor known AD susceptibility variants. We validated 11 of the differentially methylated regions in an independent set of 117 subjects. Furthermore, we functionally validated these CpG associations and identified the nearby genes whose RNA expression was altered in AD: ANK1, CDH23, DIP2A, RHBDF2, RPL13, SERPINF1 and SERPINF2. Our analyses suggest that these DNA methylation changes may have a role in the onset of AD given that we observed them in presymptomatic subjects and that six of the validated genes connect to a known AD susceptibility gene network.
Journal: Nature Neuroscience, 2014 Sep DOI: 10.1038/nn.3786 PMID: 25129075.0 PMCID: PMC4292795
122. Comparative analysis of metazoan chromatin organization.
Ho, Jung, Liu, Alver, Lee, Ikegami, Sohn, Minoda, Tolstorukov, Appert, Parker, Gu, Kundaje, Riddle, Bishop, Egelhofer, Hu, Alekseyenko, Rechtsteiner, Asker, Belsky, Bowman, Chen, Chen, Day, Dong, Dose, Duan, Epstein, Ercan, Feingold, Ferrari, Garrigues, Gehlenborg, Good, Haseley, He, Herrmann, Hoffman, Jeffers, Kharchenko, Kolasinska-Zwierz, Kotwaliwale, Kumar, Langley, Larschan, Latorre, Libbrecht, Lin, Park, Pazin, Pham, Plachetka, Qin, Schwartz, Shoresh, Stempor, Vielle, Wang, Whittle, Xue, Kingston, Kim, Bernstein, Dernburg, Pirrotta, Kuroda, Noble, Tullius, Kellis, MacAlpine, Strome, Elgin, Liu, Lieb, Ahringer, Karpen, Park
Genome function is dynamically regulated in part by chromatin, which consists of the histones, non-histone proteins and RNA molecules that package DNA. Studies in Caenorhabditis elegans and Drosophila melanogaster have contributed substantially to our understanding of molecular mechanisms of genome function in humans, and have revealed conservation of chromatin components and mechanisms. Nevertheless, the three organisms have markedly different genome sizes, chromosome architecture and gene organization. On human and fly chromosomes, for example, pericentric heterochromatin flanks single centromeres, whereas worm chromosomes have dispersed heterochromatin-like regions enriched in the distal chromosomal 'arms', and centromeres distributed along their lengths. To systematically investigate chromatin organization and associated gene regulation across species, we generated and analysed a large collection of genome-wide chromatin data sets from cell lines and developmental stages in worm, fly and human. Here we present over 800 new data sets from our ENCODE and modENCODE consortia, bringing the total to over 1,400. Comparison of combinatorial patterns of histone modifications, nuclear lamina-associated domains, organization of large-scale topological domains, chromatin environment at promoters and enhancers, nucleosome positioning, and DNA replication patterns reveals many conserved features of chromatin organization among the three organisms. We also find notable differences in the composition and locations of repressive chromatin. These data sets and analyses provide a rich resource for comparative and species-specific investigations of chromatin composition, organization and function.
Journal: Nature, 2014 Aug 28 DOI: 10.1038/nature13415 PMID: 25164756.0 PMCID: PMC4227084
121. Comparative analysis of regulatory information and circuits across distant species.
Boyle, Araya, Brdlik, Cayting, Cheng, Cheng, Gardner, Hillier, Janette, Jiang, Kasper, Kawli, Kheradpour, Kundaje, Li, Ma, Niu, Rehm, Rozowsky, Slattery, Spokony, Terrell, Vafeados, Wang, Weisdepp, Wu, Xie, Yan, Feingold, Good, Pazin, Huang, Bickel, Brenner, Reinke, Waterston, Gerstein, White, Kellis, Snyder
Despite the large evolutionary distances between metazoan species, they can show remarkable commonalities in their biology, and this has helped to establish fly and worm as model organisms for human biology. Although studies of individual elements and factors have explored similarities in gene regulation, a large-scale comparative analysis of basic principles of transcriptional regulatory features is lacking. Here we map the genome-wide binding locations of 165 human, 93 worm and 52 fly transcription regulatory factors, generating a total of 1,019 data sets from diverse cell types, developmental stages, or conditions in the three species, of which 498 (48.9%) are presented here for the first time. We find that structural properties of regulatory networks are remarkably conserved and that orthologous regulatory factor families recognize similar binding motifs in vivo and show some similar co-associations. Our results suggest that gene-regulatory properties previously observed for individual factors are general principles of metazoan regulation that are remarkably well-preserved despite extensive functional divergence of individual network connections. The comparative maps of regulatory circuitry provided here will drive an improved understanding of the regulatory underpinnings of model organism biology and how these relate to human biology, development and disease.
Journal: Nature, 2014 Aug 28 DOI: 10.1038/nature13668 PMID: 25164757.0 PMCID: PMC4336544
120. Reply to Brunet and Doolittle: Both selected effect and causal role elements can influence human biology and disease.
Kellis, Wold, Snyder, Bernstein, Kundaje, Marinov, Ward, Birney, Crawford, Dekker, Dunham, Elnitski, Farnham, Feingold, Gerstein, Giddings, Gilbert, Gingeras, Green, Guigo, Hubbard, Kent, Lieb, Myers, Pazin, Ren, Stamatoyannopoulos, Weng, White, Hardison
Journal: Proceedings Of The National Academy Of Sciences Of The United States Of America, 2014 Aug 19 DOI: 10.1073/pnas.1410434111 PMID: 25275169.0 PMCID: PMC4143047
119. Distinct and predictive histone lysine acetylation patterns at promoters, enhancers, and gene bodies.
Rajagopal, Ernst, Ray, Wu, Zhang, Kellis, Ren
In eukaryotic cells, histone lysines are frequently acetylated. However, unlike modifications such as methylations, histone acetylation modifications are often considered redundant. As such, the functional roles of distinct histone acetylations are largely unexplored. We previously developed an algorithm RFECS to discover the most informative modifications associated with the classification or prediction of mammalian enhancers. Here, we used this tool to identify the modifications most predictive of promoters, enhancers, and gene bodies. Unexpectedly, we found that histone acetylation alone performs well in distinguishing these unique genomic regions. Further, we found the association of characteristic acetylation patterns with genic regions and association of chromatin state with splicing. Taken together, our work underscores the diverse functional roles of histone acetylation in gene regulation and provides several testable hypotheses to dissect these roles.
Journal: G3, 2014 Aug 12 DOI: 10.1534/g3.114.013565 PMID: 25122670.0 PMCID: PMC4232531
118. Evidence of efficient stop codon readthrough in four mammalian genes.
Loughran, Chou, Ivanov, Jungreis, Kellis, Kiran, Baranov, Atkins
Stop codon readthrough is used extensively by viruses to expand their gene expression. Until recent discoveries in Drosophila, only a very limited number of readthrough cases in chromosomal genes had been reported. Analysis of conserved protein coding signatures that extend beyond annotated stop codons identified potential stop codon readthrough of four mammalian genes. Here we use a modified targeted bioinformatic approach to identify a further three mammalian readthrough candidates. All seven genes were tested experimentally using reporter constructs transfected into HEK-293T cells. Four displayed efficient stop codon readthrough, and these have UGA immediately followed by CUAG. Comparative genomic analysis revealed that in the four readthrough candidates containing UGA-CUAG, this motif is conserved not only in mammals but throughout vertebrates with the first six of the seven nucleotides being universally conserved. The importance of the CUAG motif was confirmed using a systematic mutagenesis approach. One gene, OPRL1, encoding an opiate receptor, displayed extremely efficient levels of readthrough ( approximately 31%) in HEK-293T cells. Signals both 5' and 3' of the OPRL1 stop codon contribute to this high level of readthrough. The sequence UGA-CUA alone can support 1.5% readthrough, underlying its importance.
Journal: Nucleic Acids Research, 2014 Aug DOI: 10.1093/nar/gku608 PMID: 25013167.0 PMCID: PMC4132726
117. Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization.
Arking, Pulit, Crotti, van, Munroe, Koopmann, Sotoodehnia, Rossin, Morley, Wang, Johnson, Lundby, Gudbjartsson, Noseworthy, Eijgelsheim, Bradford, Tarasov, Dorr, Muller-Nurasyid, Lahtinen, Nolte, Smith, Bis, Isaacs, Newhouse, Evans, Post, Waggott, Lyytikainen, Hicks, Eisele, Ellinghaus, Hayward, Navarro, Ulivi, Tanaka, Tester, Chatel, Gustafsson, Kumari, Morris, Naluai, Padmanabhan, Kluttig, Strohmer, Panayiotou, Torres, Knoflach, Hubacek, Slowikowski, Raychaudhuri, Kumar, Harris, Launer, Shuldiner, Alonso, Bader, Ehret, Huang, Kao, Strait, Macfarlane, Brown, Caulfield, Samani, Kronenberg, Willeit, Smith, Greiser, Meyer, Werdan, Carella, Zelante, Heckbert, Psaty, Rotter, Kolcic, Polasek, Wright, Griffin, Daly, Arnar, Holm, Thorsteinsdottir, Denny, Roden, Zuvich, Emilsson, Plump, Larson, O'Donnell, Yin, Bobbo, D'Adamo, Iorio, Sinagra, Carracedo, Cummings, Nalls, Jula, Kontula, Marjamaa, Oikarinen, Perola, Porthan, Erbel, Hoffmann, Jockel, Kalsch, Nothen, den, Loos, Thelle, Gieger, Meitinger, Perz, Peters, Prucha, Sinner, Waldenberger, de, Franke, van, Beckmann, Martens, Bardai, Hofman, Wilde, Behr, Dalageorgou, Giudicessi, Medeiros-Domingo, Barc, Kyndt, Probst, Ghidoni, Insolia, Hamilton, Scherer, Brandimarto, Margulies, Moravec, del, Fuchsberger, O'Connell, Lee, Watt, Campbell, Wild, El, Frey, Asselbergs, Mateo, Navis, van, van, Kellis, Krijthe, Franco, Hofman, Kors, Uitterlinden, Witteman, Kedenko, Lamina, Oostra, Abecasis, Lakatta, Mulas, Orru, Schlessinger, Uda, Markus, Volker, Snieder, Spector, Arnlov, Lind, Sundstrom, Syvanen, Kivimaki, Kahonen, Mononen, Raitakari, Viikari, Adamkova, Kiechl, Brion, Nicolaides, Paulweber, Haerting, Dominiczak, Nyberg, Whincup, Hingorani, Schott, Bezzina, Ingelsson, Ferrucci, Gasparini, Wilson, Rudan, Franke, Muhleisen, Pramstaller, Lehtimaki, Paterson, Parsa, Liu, van, Siscovick, Gudnason, Jamshidi, Salomaa, Felix, Sanna, Ritchie, Stricker, Stefansson, Boyer, Cappola, Olsen, Lage, Schwartz, Kaab, Chakravarti, Ackerman, Pfeufer, de, Newton-Cheh
The QT interval, an electrocardiographic measure reflecting myocardial repolarization, is a heritable trait. QT prolongation is a risk factor for ventricular arrhythmias and sudden cardiac death (SCD) and could indicate the presence of the potentially lethal mendelian long-QT syndrome (LQTS). Using a genome-wide association and replication study in up to 100,000 individuals, we identified 35 common variant loci associated with QT interval that collectively explain approximately 8-10% of QT-interval variation and highlight the importance of calcium regulation in myocardial repolarization. Rare variant analysis of 6 new QT interval-associated loci in 298 unrelated probands with LQTS identified coding variants not found in controls but of uncertain causality and therefore requiring validation. Several newly identified loci encode proteins that physically interact with other recognized repolarization proteins. Our integration of common variant association, expression and orthogonal protein-protein interaction screens provides new insights into cardiac electrophysiology and identifies new candidate genes for ventricular arrhythmias, LQTS and SCD.
Journal: Nature Genetics, 2014 Aug DOI: 10.1038/ng.3014 PMID: 24952745.0 PMCID: PMC4124521
116. Diverse patterns of genomic targeting by transcriptional regulators in Drosophila melanogaster.
Slattery, Ma, Spokony, Arthur, Kheradpour, Kundaje, Negre, Crofts, Ptashkin, Zieba, Ostapenko, Suchy, Victorsen, Jameel, Grundstad, Gao, Moran, Rehm, Grossman, Kellis, White
Annotation of regulatory elements and identification of the transcription-related factors (TRFs) targeting these elements are key steps in understanding how cells interpret their genetic blueprint and their environment during development, and how that process goes awry in the case of disease. One goal of the modENCODE (model organism ENCyclopedia of DNA Elements) Project is to survey a diverse sampling of TRFs, both DNA-binding and non-DNA-binding factors, to provide a framework for the subsequent study of the mechanisms by which transcriptional regulators target the genome. Here we provide an updated map of the Drosophila melanogaster regulatory genome based on the location of 84 TRFs at various stages of development. This regulatory map reveals a variety of genomic targeting patterns, including factors with strong preferences toward proximal promoter binding, factors that target intergenic and intronic DNA, and factors with distinct chromatin state preferences. The data also highlight the stringency of the Polycomb regulatory network, and show association of the Trithorax-like (Trl) protein with hotspots of DNA binding throughout development. Furthermore, the data identify more than 5800 instances in which TRFs target DNA regions with demonstrated enhancer activity. Regions of high TRF co-occupancy are more likely to be associated with open enhancers used across cell types, while lower TRF occupancy regions are associated with complex enhancers that are also regulated at the epigenetic level. Together these data serve as a resource for the research community in the continued effort to dissect transcriptional regulatory mechanisms directing Drosophila development.
Journal: Genome Research, 2014 Jul DOI: 10.1101/gr.168807.113 PMID: 24985916.0 PMCID: PMC4079976
115. Comparative validation of the D. melanogaster modENCODE transcriptome annotation.
Chen, Sturgill, Qu, Jiang, Park, Boley, Suzuki, Fletcher, Plachetzki, FitzGerald, Artieri, Atallah, Barmina, Brown, Blankenburg, Clough, Dasgupta, Gubbala, Han, Jayaseelan, Kalra, Kim, Kovar, Lee, Li, Malley, Malone, Mathew, Mattiuzzo, Munidasa, Muzny, Ongeri, Perales, Przytycka, Pu, Robinson, Thornton, Saada, Scherer, Smith, Vinson, Warner, Worley, Wu, Zou, Cherbas, Kellis, Eisen, Piano, Kionte, Fitch, Sternberg, Cutter, Duff, Hoskins, Graveley, Gibbs, Bickel, Kopp, Carninci, Celniker, Oliver, Richards
Accurate gene model annotation of reference genomes is critical for making them useful. The modENCODE project has improved the D. melanogaster genome annotation by using deep and diverse high-throughput data. Since transcriptional activity that has been evolutionarily conserved is likely to have an advantageous function, we have performed large-scale interspecific comparisons to increase confidence in predicted annotations. To support comparative genomics, we filled in divergence gaps in the Drosophila phylogeny by generating draft genomes for eight new species. For comparative transcriptome analysis, we generated mRNA expression profiles on 81 samples from multiple tissues and developmental stages of 15 Drosophila species, and we performed cap analysis of gene expression in D. melanogaster and D. pseudoobscura. We also describe conservation of four distinct core promoter structures composed of combinations of elements at three positions. Overall, each type of genomic feature shows a characteristic divergence rate relative to neutral models, highlighting the value of multispecies alignment in annotating a target genome that should prove useful in the annotation of other high priority genomes, especially human and other mammalian genomes that are rich in noncoding sequences. We report that the vast majority of elements in the annotation are evolutionarily conserved, indicating that the annotation will be an important springboard for functional genetic testing by the Drosophila community.
Journal: Genome Research, 2014 Jul DOI: 10.1101/gr.159384.113 PMID: 24985915.0 PMCID: PMC4079975
114. Pareto-optimal phylogenetic tree reconciliation.
Libeskind-Hadas, Wu, Bansal, Kellis
Phylogenetic tree reconciliation is a widely used method for reconstructing the evolutionary histories of gene families and species, hosts and parasites and other dependent pairs of entities. Reconciliation is typically performed using maximum parsimony, in which each evolutionary event type is assigned a cost and the objective is to find a reconciliation of minimum total cost. It is generally understood that reconciliations are sensitive to event costs, but little is understood about the relationship between event costs and solutions. Moreover, choosing appropriate event costs is a notoriously difficult problem. RESULTS: We address this problem by giving an efficient algorithm for computing Pareto-optimal sets of reconciliations, thus providing the first systematic method for understanding the relationship between event costs and reconciliations. This, in turn, results in new techniques for computing event support values and, for cophylogenetic analyses, performing robust statistical tests. We provide new software tools and demonstrate their use on a number of datasets from evolutionary genomic and cophylogenetic studies. AVAILABILITY AND IMPLEMENTATION: Our Python tools are freely available at www.cs.hmc.edu/ approximately hadas/xscape. .
Journal: Bioinformatics, 2014 Jun 15 DOI: 10.1093/bioinformatics/btu289 PMID: 24932009.0 PMCID: PMC4058917
113. RECOMB/ISCB systems biology, regulatory genomics, and DREAM 2013 special issue.
Califano, Kellis, Stolovitzky
Journal: Journal Of Computational Biology, 2014 May DOI: 10.1089/cmb.2014.009p PMID: 24784777.0
112. Defining functional DNA elements in the human genome.
Kellis, Wold, Snyder, Bernstein, Kundaje, Marinov, Ward, Birney, Crawford, Dekker, Dunham, Elnitski, Farnham, Feingold, Gerstein, Giddings, Gilbert, Gingeras, Green, Guigo, Hubbard, Kent, Lieb, Myers, Pazin, Ren, Stamatoyannopoulos, Weng, White, Hardison
With the completion of the human genome sequence, attention turned to identifying and annotating its functional DNA elements. As a complement to genetic and comparative genomics approaches, the Encyclopedia of DNA Elements Project was launched to contribute maps of RNA transcripts, transcriptional regulator binding sites, and chromatin states in many cell types. The resulting genome-wide data reveal sites of biochemical activity with high positional resolution and cell type specificity that facilitate studies of gene regulation and interpretation of noncoding variants associated with human disease. However, the biochemically active regions cover a much larger fraction of the genome than do evolutionarily conserved regions, raising the question of whether nonconserved but biochemically active regions are truly functional. Here, we review the strengths and limitations of biochemical, evolutionary, and genetic approaches for defining functional DNA segments, potential sources for the observed differences in estimated genomic coverage, and the biological implications of these discrepancies. We also analyze the relationship between signal intensity, genomic coverage, and evolutionary conservation. Our results reinforce the principle that each approach provides complementary information and that we need to use combinations of all three to elucidate genome function in human biology and disease.
Journal: Proceedings Of The National Academy Of Sciences Of The United States Of America, 2014 Apr 29 DOI: 10.1073/pnas.1318948111 PMID: 24753594.0 PMCID: PMC4035993
111. Evolutionary dynamics and tissue specificity of human long noncoding RNAs in six mammals.
Washietl, Kellis, Garber
Long intergenic noncoding RNAs (lincRNAs) play diverse regulatory roles in human development and disease, but little is known about their evolutionary history and constraint. Here, we characterize human lincRNA expression patterns in nine tissues across six mammalian species and multiple individuals. Of the 1898 human lincRNAs expressed in these tissues, we find orthologous transcripts for 80% in chimpanzee, 63% in rhesus, 39% in cow, 38% in mouse, and 35% in rat. Mammalian-expressed lincRNAs show remarkably strong conservation of tissue specificity, suggesting that it is selectively maintained. In contrast, abundant splice-site turnover suggests that exact splice sites are not critical. Relative to evolutionarily young lincRNAs, mammalian-expressed lincRNAs show higher primary sequence conservation in their promoters and exons, increased proximity to protein-coding genes enriched for tissue-specific functions, fewer repeat elements, and more frequent single-exon transcripts. Remarkably, we find that approximately 20% of human lincRNAs are not expressed beyond chimpanzee and are undetectable even in rhesus. These hominid-specific lincRNAs are more tissue specific, enriched for testis, and faster evolving within the human lineage.
Journal: Genome Research, 2014 Apr DOI: 10.1101/gr.165035.113 PMID: 24429298.0 PMCID: PMC3975061
110. Common genetic variants modulate pathogen-sensing responses in human dendritic cells.
Lee, Ye, Villani, Raj, Li, Eisenhaure, Imboywa, Chipendo, Ran, Slowikowski, Ward, Raddassi, McCabe, Lee, Frohlich, Hafler, Kellis, Raychaudhuri, Zhang, Stranger, Benoist, De, Regev, Hacohen
Little is known about how human genetic variation affects the responses to environmental stimuli in the context of complex diseases. Experimental and computational approaches were applied to determine the effects of genetic variation on the induction of pathogen-responsive genes in human dendritic cells. We identified 121 common genetic variants associated in cis with variation in expression responses to Escherichia coli lipopolysaccharide, influenza, or interferon-beta (IFN-beta). We localized and validated causal variants to binding sites of pathogen-activated STAT (signal transducer and activator of transcription) and IRF (IFN-regulatory factor) transcription factors. We also identified a common variant in IRF7 that is associated in trans with type I IFN induction in response to influenza infection. Our results reveal common alleles that explain interindividual variation in pathogen sensing and provide functional annotation for genetic variants that alter susceptibility to inflammatory diseases.
Journal: Science, 2014 Mar 7 DOI: 10.1126/science.1246980 PMID: 24604203.0 PMCID: PMC4124741
109. Discovery of human sORF-encoded polypeptides (SEPs) in cell lines and tissue.
Ma, Ward, Jungreis, Slavoff, Schwaid, Neveu, Budnik, Kellis, Saghatelian
The existence of nonannotated protein-coding human short open reading frames (sORFs) has been revealed through the direct detection of their sORF-encoded polypeptide (SEP) products. The discovery of novel SEPs increases the size of the genome and the proteome and provides insights into the molecular biology of mammalian cells, such as the prevalent usage of non-AUG start codons. Through modifications of the existing SEP-discovery workflow, we discover an additional 195 SEPs in K562 cells and extend this methodology to identify novel human SEPs in additional cell lines and human tissue for a final tally of 237 new SEPs. These results continue to expand the human genome and proteome and demonstrate that SEPs are a ubiquitous class of nonannotated polypeptides that require further investigation.
Journal: Journal Of Proteome Research, 2014 Mar 7 DOI: 10.1021/pr401280w PMID: 24490786.0 PMCID: PMC3993966
108. Systematic discovery and characterization of regulatory motifs in ENCODE TF binding experiments.
Kheradpour, Kellis
Recent advances in technology have led to a dramatic increase in the number of available transcription factor ChIP-seq and ChIP-chip data sets. Understanding the motif content of these data sets is an important step in understanding the underlying mechanisms of regulation. Here we provide a systematic motif analysis for 427 human ChIP-seq data sets using motifs curated from the literature and also discovered de novo using five established motif discovery tools. We use a systematic pipeline for calculating motif enrichment in each data set, providing a principled way for choosing between motif variants found in the literature and for flagging potentially problematic data sets. Our analysis confirms the known specificity of 41 of the 56 analyzed factor groups and reveals motifs of potential cofactors. We also use cell type-specific binding to find factors active in specific conditions. The resource we provide is accessible both for browsing a small number of factors and for performing large-scale systematic analyses. We provide motif matrices, instances and enrichments in each of the ENCODE data sets. The motifs discovered here have been used in parallel studies to validate the specificity of antibodies, understand cooperativity between data sets and measure the variation of motif binding across individuals and species.
Journal: Nucleic Acids Research, 2014 Mar DOI: 10.1093/nar/gkt1249 PMID: 24335146.0 PMCID: PMC3950668
107. Most parsimonious reconciliation in the presence of gene duplication, loss, and deep coalescence using labeled coalescent trees.
Wu, Rasmussen, Bansal, Kellis
Accurate gene tree-species tree reconciliation is fundamental to inferring the evolutionary history of a gene family. However, although it has long been appreciated that population-related effects such as incomplete lineage sorting (ILS) can dramatically affect the gene tree, many of the most popular reconciliation methods consider discordance only due to gene duplication and loss (and sometimes horizontal gene transfer). Methods that do model ILS are either highly parameterized or consider a restricted set of histories, thus limiting their applicability and accuracy. To address these challenges, we present a novel algorithm DLCpar for inferring a most parsimonious (MP) history of a gene family in the presence of duplications, losses, and ILS. Our algorithm relies on a new reconciliation structure, the labeled coalescent tree (LCT), that simultaneously describes coalescent and duplication-loss history. We show that the LCT representation enables an exhaustive and efficient search over the space of reconciliations, and, for most gene families, the least common ancestor (LCA) mapping is an optimal solution for the species mapping between the gene tree and species tree in an MP LCT. Applying our algorithm to a variety of clades, including flies, fungi, and primates, as well as to simulated phylogenies, we achieve high accuracy, comparable to sophisticated probabilistic reconciliation methods, at reduced run time and with far fewer parameters. These properties enable inferences of the complex evolution of gene families across a broad range of species and large data sets.
Journal: Genome Research, 2014 Mar DOI: 10.1101/gr.161968.113 PMID: 24310000.0 PMCID: PMC3941112
106. Genome-wide probing of RNA structure reveals active unfolding of mRNA structures in vivo.
Rouskin, Zubradt, Washietl, Kellis, Weissman
RNA has a dual role as an informational molecule and a direct effector of biological tasks. The latter function is enabled by RNA's ability to adopt complex secondary and tertiary folds and thus has motivated extensive computational and experimental efforts for determining RNA structures. Existing approaches for evaluating RNA structure have been largely limited to in vitro systems, yet the thermodynamic forces which drive RNA folding in vitro may not be sufficient to predict stable RNA structures in vivo. Indeed, the presence of RNA-binding proteins and ATP-dependent helicases can influence which structures are present inside cells. Here we present an approach for globally monitoring RNA structure in native conditions in vivo with single-nucleotide precision. This method is based on in vivo modification with dimethyl sulphate (DMS), which reacts with unpaired adenine and cytosine residues, followed by deep sequencing to monitor modifications. Our data from yeast and mammalian cells are in excellent agreement with known messenger RNA structures and with the high-resolution crystal structure of the Saccharomyces cerevisiae ribosome. Comparison between in vivo and in vitro data reveals that in rapidly dividing cells there are vastly fewer structured mRNA regions in vivo than in vitro. Even thermostable RNA structures are often denatured in cells, highlighting the importance of cellular processes in regulating RNA structure. Indeed, analysis of mRNA structure under ATP-depleted conditions in yeast shows that energy-dependent processes strongly contribute to the predominantly unfolded state of mRNAs inside cells. Our studies broadly enable the functional analysis of physiological RNA structures and reveal that, in contrast to the Anfinsen view of protein folding whereby the structure formed is the most thermodynamically favourable, thermodynamics have an incomplete role in determining mRNA structure in vivo.
Journal: Nature, 2014 Jan 30 DOI: 10.1038/nature12894 PMID: 24336214.0 PMCID: PMC3966492
105. Energy-based RNA consensus secondary structure prediction in multiple sequence alignments.
Washietl, Bernhart, Kellis
Many biologically important RNA structures are conserved in evolution leading to characteristic mutational patterns. RNAalifold is a widely used program to predict consensus secondary structures in multiple alignments by combining evolutionary information with traditional energy-based RNA folding algorithms. Here we describe the theory and applications of the RNAalifold algorithm. Consensus secondary structure prediction not only leads to significantly more accurate structure models, but it also allows to study structural conservation of functional RNAs.
Journal: Methods In Molecular Biology, 2014 DOI: 10.1007/978-1-62703-709-9_7 PMID: 24639158.0
104. Multiple knockout mouse models reveal lincRNAs are required for life and brain development.
Sauvageau, Goff, Lodato, Bonev, Groff, Gerhardinger, Sanchez-Gomez, Hacisuleyman, Li, Spence, Liapis, Mallard, Morse, Swerdel, D'Ecclessis, Moore, Lai, Gong, Yancopoulos, Frendewey, Kellis, Hart, Valenzuela, Arlotta, Rinn
Many studies are uncovering functional roles for long noncoding RNAs (lncRNAs), yet few have been tested for in vivo relevance through genetic ablation in animal models. To investigate the functional relevance of lncRNAs in various physiological conditions, we have developed a collection of 18 lncRNA knockout strains in which the locus is maintained transcriptionally active. Initial characterization revealed peri- and postnatal lethal phenotypes in three mutant strains (Fendrr, Peril, and Mdgt), the latter two exhibiting incomplete penetrance and growth defects in survivors. We also report growth defects for two additional mutant strains (linc-Brn1b and linc-Pint). Further analysis revealed defects in lung, gastrointestinal tract, and heart in Fendrr(-/-) neonates, whereas linc-Brn1b(-/-) mutants displayed distinct abnormalities in the generation of upper layer II-IV neurons in the neocortex. This study demonstrates that lncRNAs play critical roles in vivo and provides a framework and impetus for future larger-scale functional investigation into the roles of lncRNA molecules. DOI: http://dx.doi.org/10.7554/eLife.01749.001.
Journal: Elife, 2013 Dec 31 DOI: 10.7554/eLife.01749 PMID: 24381249.0 PMCID: PMC3874104
103. Spatial expression of transcription factors in Drosophila embryonic organ development.
Hammonds, Bristow, Fisher, Weiszmann, Wu, Hartenstein, Kellis, Yu, Frise, Celniker
Site-specific transcription factors (TFs) bind DNA regulatory elements to control expression of target genes, forming the core of gene regulatory networks. Despite decades of research, most studies focus on only a small number of TFs and the roles of many remain unknown. RESULTS: We present a systematic characterization of spatiotemporal gene expression patterns for all known or predicted Drosophila TFs throughout embryogenesis, the first such comprehensive study for any metazoan animal. We generated RNA expression patterns for all 708 TFs by in situ hybridization, annotated the patterns using an anatomical controlled vocabulary, and analyzed TF expression in the context of organ system development. Nearly all TFs are expressed during embryogenesis and more than half are specifically expressed in the central nervous system. Compared to other genes, TFs are enriched early in the development of most organ systems, and throughout the development of the nervous system. Of the 535 TFs with spatially restricted expression, 79% are dynamically expressed in multiple organ systems while 21% show single-organ specificity. Of those expressed in multiple organ systems, 77 TFs are restricted to a single organ system either early or late in development. Expression patterns for 354 TFs are characterized for the first time in this study. CONCLUSIONS: We produced a reference TF dataset for the investigation of gene regulatory networks in embryogenesis, and gained insight into the expression dynamics of the full complement of TFs controlling the development of each organ system.
Journal: Genome Biology, 2013 Dec 20 DOI: 10.1186/gb-2013-14-12-r140 PMID: 24359758.0 PMCID: PMC4053779
102. Integrating and mining the chromatin landscape of cell-type specificity using self-organizing maps.
Mortazavi, Pepke, Jansen, Marinov, Ernst, Kellis, Hardison, Myers, Wold
We tested whether self-organizing maps (SOMs) could be used to effectively integrate, visualize, and mine diverse genomics data types, including complex chromatin signatures. A fine-grained SOM was trained on 72 ChIP-seq histone modifications and DNase-seq data sets from six biologically diverse cell lines studied by The ENCODE Project Consortium. We mined the resulting SOM to identify chromatin signatures related to sequence-specific transcription factor occupancy, sequence motif enrichment, and biological functions. To highlight clusters enriched for specific functions such as transcriptional promoters or enhancers, we overlaid onto the map additional data sets not used during training, such as ChIP-seq, RNA-seq, CAGE, and information on cis-acting regulatory modules from the literature. We used the SOM to parse known transcriptional enhancers according to the cell-type-specific chromatin signature, and we further corroborated this pattern on the map by EP300 (also known as p300) occupancy. New candidate cell-type-specific enhancers were identified for multiple ENCODE cell types in this way, along with new candidates for ubiquitous enhancer activity. An interactive web interface was developed to allow users to visualize and custom-mine the ENCODE SOM. We conclude that large SOMs trained on chromatin data from multiple cell types provide a powerful way to identify complex relationships in genomic data at user-selected levels of granularity.
Journal: Genome Research, 2013 Dec DOI: 10.1101/gr.158261.113 PMID: 24170599.0 PMCID: PMC3847782
101. Extensive variation in chromatin states across humans.
Kasowski, Kyriazopoulou-Panagiotopoulou, Grubert, Zaugg, Kundaje, Liu, Boyle, Zhang, Zakharia, Spacek, Li, Xie, Olarerin-George, Steinmetz, Hogenesch, Kellis, Batzoglou, Snyder
The majority of disease-associated variants lie outside protein-coding regions, suggesting a link between variation in regulatory regions and disease predisposition. We studied differences in chromatin states using five histone modifications, cohesin, and CTCF in lymphoblastoid lines from 19 individuals of diverse ancestry. We found extensive signal variation in regulatory regions, which often switch between active and repressed states across individuals. Enhancer activity is particularly diverse among individuals, whereas gene expression remains relatively stable. Chromatin variability shows genetic inheritance in trios, correlates with genetic variation and population divergence, and is associated with disruptions of transcription factor binding motifs. Overall, our results provide insights into chromatin variation among humans.
Journal: Science, 2013 Nov 8 DOI: 10.1126/science.1242510 PMID: 24136358.0 PMCID: PMC4075767
100. Reconciliation revisited: handling multiple optima when reconciling with duplication, transfer, and loss.
Bansal, Alm, Kellis
Phylogenetic tree reconciliation is a powerful approach for inferring evolutionary events like gene duplication, horizontal gene transfer, and gene loss, which are fundamental to our understanding of molecular evolution. While duplication-loss (DL) reconciliation leads to a unique maximum-parsimony solution, duplication-transfer-loss (DTL) reconciliation yields a multitude of optimal solutions, making it difficult to infer the true evolutionary history of the gene family. This problem is further exacerbated by the fact that different event cost assignments yield different sets of optimal reconciliations. Here, we present an effective, efficient, and scalable method for dealing with these fundamental problems in DTL reconciliation. Our approach works by sampling the space of optimal reconciliations uniformly at random and aggregating the results. We show that even gene trees with only a few dozen genes often have millions of optimal reconciliations and present an algorithm to efficiently sample the space of optimal reconciliations uniformly at random in O(mn(2)) time per sample, where m and n denote the number of genes and species, respectively. We use these samples to understand how different optimal reconciliations vary in their node mappings and event assignments and to investigate the impact of varying event costs. We apply our method to a biological dataset of approximately 4700 gene trees from 100 taxa and observe that 93% of event assignments and 73% of mappings remain consistent across different multiple optima. Our analysis represents the first systematic investigation of the space of optimal DTL reconciliations and has many important implications for the study of gene family evolution.
Journal: Journal Of Computational Biology, 2013 Oct DOI: 10.1089/cmb.2013.0073 PMID: 24033262.0 PMCID: PMC3791060
99. Genomic evidence for ameiotic evolution in the bdelloid rotifer Adineta vaga.
Flot, Hespeels, Li, Noel, Arkhipova, Danchin, Hejnol, Henrissat, Koszul, Aury, Barbe, Barthelemy, Bast, Bazykin, Chabrol, Couloux, Da, Da, Gladyshev, Gouret, Hallatschek, Hecox-Lea, Labadie, Lejeune, Piskurek, Poulain, Rodriguez, Ryan, Vakhrusheva, Wajnberg, Wirth, Yushenova, Kellis, Kondrashov, Mark, Pontarotti, Weissenbach, Wincker, Jaillon, Van
Loss of sexual reproduction is considered an evolutionary dead end for metazoans, but bdelloid rotifers challenge this view as they appear to have persisted asexually for millions of years. Neither male sex organs nor meiosis have ever been observed in these microscopic animals: oocytes are formed through mitotic divisions, with no reduction of chromosome number and no indication of chromosome pairing. However, current evidence does not exclude that they may engage in sex on rare, cryptic occasions. Here we report the genome of a bdelloid rotifer, Adineta vaga (Davis, 1873), and show that its structure is incompatible with conventional meiosis. At gene scale, the genome of A. vaga is tetraploid and comprises both anciently duplicated segments and less divergent allelic regions. However, in contrast to sexual species, the allelic regions are rearranged and sometimes even found on the same chromosome. Such structure does not allow meiotic pairing; instead, we find abundant evidence of gene conversion, which may limit the accumulation of deleterious mutations in the absence of meiosis. Gene families involved in resistance to oxidation, carbohydrate metabolism and defence against transposons are significantly expanded, which may explain why transposable elements cover only 3% of the assembled sequence. Furthermore, 8% of the genes are likely to be of non-metazoan origin and were probably acquired horizontally. This apparent convergence between bdelloids and prokaryotes sheds new light on the evolutionary significance of sex.
Journal: Nature, 2013 Aug 22 DOI: 10.1038/nature12326 PMID: 23873043.0
98. Network deconvolution as a general method to distinguish direct dependencies in networks.
Feizi, Marbach, Medard, Kellis
Recognizing direct relationships between variables connected in a network is a pervasive problem in biological, social and information sciences as correlation-based networks contain numerous indirect relationships. Here we present a general method for inferring direct effects from an observed correlation matrix containing both direct and indirect effects. We formulate the problem as the inverse of network convolution, and introduce an algorithm that removes the combined effect of all indirect paths of arbitrary length in a closed-form solution by exploiting eigen-decomposition and infinite-series sums. We demonstrate the effectiveness of our approach in several network applications: distinguishing direct targets in gene expression regulatory networks; recognizing directly interacting amino-acid residues for protein structure prediction from sequence alignments; and distinguishing strong collaborations in co-authorship social networks using connectivity information alone. In addition to its theoretical impact as a foundational graph theoretic tool, our results suggest network deconvolution is widely applicable for computing direct dependencies in network science across diverse disciplines.
Journal: Nature Biotechnology, 2013 Aug DOI: 10.1038/nbt.2635 PMID: 23851448.0 PMCID: PMC3773370
97. Correction: Evolutionary principles of modular gene regulation in yeasts.
Thompson, Roy, Chan, Styczynski, Pfiffner, French, Socha, Thielke, Napolitano, Muller, Kellis, Konieczka, Wapinski, Regev
[This corrects the article DOI: 10.7554/eLife.00603.].
Journal: Elife, 2013 Jul 2 DOI: 10.7554/eLife.01114 PMID: 23840936.0 PMCID: PMC3699816
96. Interplay between chromatin state, regulator binding, and regulatory motifs in six human cell types.
Ernst, Kellis
The regions bound by sequence-specific transcription factors can be highly variable across different cell types despite the static nature of the underlying genome sequence. This has been partly attributed to changes in chromatin accessibility, but a systematic picture has been hindered by the lack of large-scale data sets. Here, we use 456 binding experiments for 119 regulators and 84 chromatin maps generated by the ENCODE in six human cell types, and relate those to a global map of regulatory motif instances for these factors. We find specific and robust chromatin state preferences for each regulator beyond the previously reported open-chromatin association, suggesting a much richer chromatin landscape beyond simple accessibility. The preferentially bound chromatin states of regulators were enriched for sequence motifs of regulators relative to all states, suggesting that these preferences are at least partly encoded by the genomic sequence. Relative to all regions bound by a regulator, however, regulatory motifs were surprisingly depleted in the regulator's preferentially bound states, suggesting additional non-sequence-specific binding beyond the level predicted by the regulatory motifs. Such permissive binding was largely restricted to open-chromatin regions showing histone modification marks characteristic of active enhancer and promoter regions, whereas open-chromatin regions lacking such marks did not show permissive binding. Lastly, the vast majority of cobinding of regulator pairs is predicted by the chromatin state preferences of individual regulators. Overall, our results suggest a joint role of sequence motifs and specific chromatin states beyond mere accessibility in mediating regulator binding dynamics across different cell types.
Journal: Genome Research, 2013 Jul DOI: 10.1101/gr.144840.112 PMID: 23595227.0 PMCID: PMC3698507
95. Evolutionary principles of modular gene regulation in yeasts.
Thompson, Roy, Chan, Styczynsky, Pfiffner, French, Socha, Thielke, Napolitano, Muller, Kellis, Konieczka, Wapinski, Regev
Divergence in gene regulation can play a major role in evolution. Here, we used a phylogenetic framework to measure mRNA profiles in 15 yeast species from the phylum Ascomycota and reconstruct the evolution of their modular regulatory programs along a time course of growth on glucose over 300 million years [corrected]. We found that modules have diverged proportionally to phylogenetic distance, with prominent changes in gene regulation accompanying changes in lifestyle and ploidy, especially in carbon metabolism. Paralogs have significantly contributed to regulatory divergence, typically within a very short window from their duplication. Paralogs from a whole genome duplication (WGD) event have a uniquely substantial contribution that extends over a longer span. Similar patterns occur when considering the evolution of the heat shock regulatory program measured in eight of the species, suggesting that these are general evolutionary principles. DOI:http://dx.doi.org/10.7554/eLife.00603.001.
Journal: Elife, 2013 Jun 18 DOI: 10.7554/eLife.00603 PMID: 23795289.0 PMCID: PMC3687341
94. The Genotype-Tissue Expression (GTEx) project.
The GTEx Consortium
Genome-wide association studies have identified thousands of loci for common diseases, but, for the majority of these, the mechanisms underlying disease susceptibility remain unknown. Most associated variants are not correlated with protein-coding changes, suggesting that polymorphisms in regulatory regions probably contribute to many disease phenotypes. Here we describe the Genotype-Tissue Expression (GTEx) project, which will establish a resource database and associated tissue bank for the scientific community to study the relationship between genetic variation and gene expression in human tissues.
Journal: Nature Genetics, 2013 Jun DOI: 10.1038/ng.2653 PMID: 23715323.0 PMCID: PMC4010069
93. Arboretum: reconstruction and analysis of the evolutionary history of condition-specific transcriptional modules.
Roy, Wapinski, Pfiffner, French, Socha, Konieczka, Habib, Kellis, Thompson, Regev
Comparative functional genomics studies the evolution of biological processes by analyzing functional data, such as gene expression profiles, across species. A major challenge is to compare profiles collected in a complex phylogeny. Here, we present Arboretum, a novel scalable computational algorithm that integrates expression data from multiple species with species and gene phylogenies to infer modules of coexpressed genes in extant species and their evolutionary histories. We also develop new, generally applicable measures of conservation and divergence in gene regulatory modules to assess the impact of changes in gene content and expression on module evolution. We used Arboretum to study the evolution of the transcriptional response to heat shock in eight species of Ascomycota fungi and to reconstruct modules of the ancestral environmental stress response (ESR). We found substantial conservation in the stress response across species and in the reconstructed components of the ancestral ESR modules. The greatest divergence was in the most induced stress, primarily through module expansion. The divergence of the heat stress response exceeds that observed in the response to glucose depletion in the same species. Arboretum and its associated analyses provide a comprehensive framework to systematically study regulatory evolution of condition-specific responses.
Journal: Genome Research, 2013 Jun DOI: 10.1101/gr.146233.112 PMID: 23640720.0 PMCID: PMC3668358
92. Response to comment on "Evidence of abundant purifying selection in humans for recently acquired regulatory functions".
Ward, Kellis
Green and Ewing propose corrections to our methodology, which we incorporate and extend here. The improved methodology supports our initial conclusion of extensive lineage-specific constraint concentrated in ENCODE elements. We clarify that our estimate is dependent on the constrained and neutral references used, which can further increase the number of nucleotides involved, because a particularly stringent definition was initially used.
Journal: Science, 2013 May 10 DOI: 10.1126/science.1233366 PMID: 23661743.0 PMCID: PMC4131756
91. Systematic dissection of regulatory motifs in 2000 predicted human enhancers using a massively parallel reporter assay.
Kheradpour, Ernst, Melnikov, Rogov, Wang, Zhang, Alston, Mikkelsen, Kellis
Genome-wide chromatin annotations have permitted the mapping of putative regulatory elements across multiple human cell types. However, their experimental dissection by directed regulatory motif disruption has remained unfeasible at the genome scale. Here, we use a massively parallel reporter assay (MPRA) to measure the transcriptional levels induced by 145-bp DNA segments centered on evolutionarily conserved regulatory motif instances within enhancer chromatin states. We select five predicted activators (HNF1, HNF4, FOXA, GATA, NFE2L2) and two predicted repressors (GFI1, ZFP161) and measure reporter expression in erythroleukemia (K562) and liver carcinoma (HepG2) cell lines. We test 2104 wild-type sequences and 3314 engineered enhancer variants containing targeted motif disruptions, each using 10 barcode tags and two replicates. The resulting data strongly confirm the enhancer activity and cell-type specificity of enhancer chromatin states, the ability of 145-bp segments to recapitulate both, the necessary role of regulatory motifs in enhancer function, and the complementary roles of activator and repressor motifs. We find statistically robust evidence that (1) disrupting the predicted activator motifs abolishes enhancer function, while silent or motif-improving changes maintain enhancer activity; (2) evolutionary conservation, nucleosome exclusion, binding of other factors, and strength of the motif match are predictive of enhancer activity; (3) scrambling repressor motifs leads to aberrant reporter expression in cell lines where the enhancers are usually inactive. Our results suggest a general strategy for deciphering cis-regulatory elements by systematic large-scale manipulation and provide quantitative enhancer activity measurements across thousands of constructs that can be mined to develop predictive models of gene expression.
Journal: Genome Research, 2013 May DOI: 10.1101/gr.144899.112 PMID: 23512712.0 PMCID: PMC3638136
90. Preface: RECOMB systems biology, regulatory genomics, and DREAM 2012 special issue.
Califano, Kellis, Stolovitzky
Journal: Journal Of Computational Biology, 2013 May DOI: 10.1089/cmb.2013.008p PMID: 23641866.0
89. Long noncoding RNAs regulate adipogenesis.
Sun, Goff, Trapnell, Alexander, Lo, Hacisuleyman, Sauvageau, Tazon-Vega, Kelley, Hendrickson, Yuan, Kellis, Lodish, Rinn
The prevalence of obesity has led to a surge of interest in understanding the detailed mechanisms underlying adipocyte development. Many protein-coding genes, mRNAs, and microRNAs have been implicated in adipocyte development, but the global expression patterns and functional contributions of long noncoding RNA (lncRNA) during adipogenesis have not been explored. Here we profiled the transcriptome of primary brown and white adipocytes, preadipocytes, and cultured adipocytes and identified 175 lncRNAs that are specifically regulated during adipogenesis. Many lncRNAs are adipose-enriched, strongly induced during adipogenesis, and bound at their promoters by key transcription factors such as peroxisome proliferator-activated receptor gamma (PPARgamma) and CCAAT/enhancer-binding protein alpha (CEBPalpha). RNAi-mediated loss of function screens identified functional lncRNAs with varying impact on adipogenesis. Collectively, we have identified numerous lncRNAs that are functionally required for proper adipogenesis.
Journal: Proceedings Of The National Academy Of Sciences Of The United States Of America, 2013 Feb 26 DOI: 10.1073/pnas.1222643110 PMID: 23401553.0 PMCID: PMC3587215
88. Constitutive nuclear lamina-genome interactions are highly conserved and associated with A/T-rich sequence.
Meuleman, Peric-Hupkes, Kind, Beaudry, Pagie, Kellis, Reinders, Wessels, van
In metazoans, the nuclear lamina is thought to play an important role in the spatial organization of interphase chromosomes, by providing anchoring sites for large genomic segments named lamina-associated domains (LADs). Some of these LADs are cell-type specific, while many others appear constitutively associated with the lamina. Constitutive LADs (cLADs) may contribute to a basal chromosome architecture. By comparison of mouse and human lamina interaction maps, we find that the sizes and genomic positions of cLADs are strongly conserved. Moreover, cLADs are depleted of synteny breakpoints, pointing to evolutionary selective pressure to keep cLADs intact. Paradoxically, the overall sequence conservation is low for cLADs. Instead, cLADs are universally characterized by long stretches of DNA of high A/T content. Cell-type specific LADs also tend to adhere to this "A/T rule" in embryonic stem cells, but not in differentiated cells. This suggests that the A/T rule represents a default positioning mechanism that is locally overruled during lineage commitment. Analysis of paralogs suggests that during evolution changes in A/T content have driven the relocation of genes to and from the nuclear lamina, in tight association with changes in expression level. Taken together, these results reveal that the spatial organization of mammalian genomes is highly conserved and tightly linked to local nucleotide composition.
Journal: Genome Research, 2013 Feb DOI: 10.1101/gr.141028.112 PMID: 23124521.0 PMCID: PMC3561868
87. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse.
Grote, Wittler, Hendrix, Koch, Wahrisch, Beisaw, Macura, Blass, Kellis, Werber, Herrmann
The histone-modifying complexes PRC2 and TrxG/MLL play pivotal roles in determining the activation state of genes controlling pluripotency, lineage commitment, and cell differentiation. Long noncoding RNAs (lncRNAs) can bind to either complex, and some have been shown to act as modulators of PRC2 or TrxG/MLL activity. Here we show that the lateral mesoderm-specific lncRNA Fendrr is essential for proper heart and body wall development in the mouse. Embryos lacking Fendrr displayed upregulation of several transcription factors controlling lateral plate or cardiac mesoderm differentiation, accompanied by a drastic reduction in PRC2 occupancy along with decreased H3K27 trimethylation and/or an increase in H3K4 trimethylation at their promoters. Fendrr binds to both the PRC2 and TrxG/MLL complexes, suggesting that it acts as modulator of chromatin signatures that define gene activity. Thus, we identified an lncRNA that plays an essential role in the regulatory networks controlling the fate of lateral mesoderm derivatives.
Journal: Developmental Cell, 2013 Jan 28 DOI: 10.1016/j.devcel.2012.12.012 PMID: 23369715.0 PMCID: PMC4149175
86. Heterologous stop codon readthrough of metazoan readthrough candidates in yeast.
Chan, Jungreis, Kellis
Recent analysis of genomic signatures in mammals, flies, and worms indicates that functional translational stop codon readthrough is considerably more abundant in metazoa than previously recognized, but this analysis provides only limited clues about the function or mechanism of readthrough. If an mRNA known to be read through in one species is also read through in another, perhaps these questions can be studied in a simpler setting. With this end in mind, we have investigated whether some of the readthrough genes in human, fly, and worm also exhibit readthrough when expressed in S. cerevisiae. We found that readthrough was highest in a gene with a post-stop hexamer known to trigger readthrough, while other metazoan readthrough genes exhibit borderline readthrough in S. cerevisiae.
Journal: Plos One, 2013 DOI: 10.1371/journal.pone.0059450 PMID: 23544069.0 PMCID: PMC3609751
85. Integrative annotation of chromatin elements from ENCODE data.
Hoffman, Ernst, Wilder, Kundaje, Harris, Libbrecht, Giardine, Ellenbogen, Bilmes, Birney, Hardison, Dunham, Kellis, Noble
The ENCODE Project has generated a wealth of experimental information mapping diverse chromatin properties in several human cell lines. Although each such data track is independently informative toward the annotation of regulatory elements, their interrelations contain much richer information for the systematic annotation of regulatory elements. To uncover these interrelations and to generate an interpretable summary of the massive datasets of the ENCODE Project, we apply unsupervised learning methodologies, converting dozens of chromatin datasets into discrete annotation maps of regulatory regions and other chromatin elements across the human genome. These methods rediscover and summarize diverse aspects of chromatin architecture, elucidate the interplay between chromatin activity and RNA transcription, and reveal that a large proportion of the genome lies in a quiescent state, even across multiple cell types. The resulting annotation of non-coding regulatory elements correlate strongly with mammalian evolutionary constraint, and provide an unbiased approach for evaluating metrics of evolutionary constraint in human. Lastly, we use the regulatory annotations to revisit previously uncharacterized disease-associated loci, resulting in focused, testable hypotheses through the lens of the chromatin landscape.
Journal: Nucleic Acids Research, 2013 Jan DOI: 10.1093/nar/gks1284 PMID: 23221638.0 PMCID: PMC3553955
84. TreeFix: statistically informed gene tree error correction using species trees.
Wu, Rasmussen, Bansal, Kellis
Accurate gene tree reconstruction is a fundamental problem in phylogenetics, with many important applications. However, sequence data alone often lack enough information to confidently support one gene tree topology over many competing alternatives. Here, we present a novel framework for combining sequence data and species tree information, and we describe an implementation of this framework in TreeFix, a new phylogenetic program for improving gene tree reconstructions. Given a gene tree (preferably computed using a maximum-likelihood phylogenetic program), TreeFix finds a "statistically equivalent" gene tree that minimizes a species tree-based cost function. We have applied TreeFix to 2 clades of 12 Drosophila and 16 fungal genomes, as well as to simulated phylogenies and show that it dramatically improves reconstructions compared with current state-of-the-art programs. Given its accuracy, speed, and simplicity, TreeFix should be applicable to a wide range of analyses and have many important implications for future investigations of gene evolution. The source code and a sample data set are available at http://compbio.mit.edu/treefix.
Journal: Systematic Biology, 2013 Jan 1 DOI: 10.1093/sysbio/sys076 PMID: 22949484.0 PMCID: PMC3526801
83. RFECS: a random-forest based algorithm for enhancer identification from chromatin state.
Rajagopal, Xie, Li, Wagner, Wang, Stamatoyannopoulos, Ernst, Kellis, Ren
Transcriptional enhancers play critical roles in regulation of gene expression, but their identification in the eukaryotic genome has been challenging. Recently, it was shown that enhancers in the mammalian genome are associated with characteristic histone modification patterns, which have been increasingly exploited for enhancer identification. However, only a limited number of cell types or chromatin marks have previously been investigated for this purpose, leaving the question unanswered whether there exists an optimal set of histone modifications for enhancer prediction in different cell types. Here, we address this issue by exploring genome-wide profiles of 24 histone modifications in two distinct human cell types, embryonic stem cells and lung fibroblasts. We developed a Random-Forest based algorithm, RFECS (Random Forest based Enhancer identification from Chromatin States) to integrate histone modification profiles for identification of enhancers, and used it to identify enhancers in a number of cell-types. We show that RFECS not only leads to more accurate and precise prediction of enhancers than previous methods, but also helps identify the most informative and robust set of three chromatin marks for enhancer prediction.
Journal: Plos Computational Biology, 2013 DOI: 10.1371/journal.pcbi.1002968 PMID: 23526891.0 PMCID: PMC3597546
82. Disruption of a large intergenic noncoding RNA in subjects with neurodevelopmental disabilities.
Talkowski, Maussion, Crapper, Rosenfeld, Blumenthal, Hanscom, Chiang, Lindgren, Pereira, Ruderfer, Diallo, Lopez, Turecki, Chen, Gigek, Harris, Lip, An, Biagioli, Macdonald, Lin, Haggarty, Sklar, Purcell, Kellis, Schwartz, Shaffer, Natowicz, Shen, Morton, Gusella, Ernst
Large intergenic noncoding (linc) RNAs represent a newly described class of ribonucleic acid whose importance in human disease remains undefined. We identified a severely developmentally delayed 16-year-old female with karyotype 46,XX,t(2;11)(p25.1;p15.1)dn in the absence of clinically significant copy number variants (CNVs). DNA capture followed by next-generation sequencing of the translocation breakpoints revealed disruption of a single noncoding gene on chromosome 2, LINC00299, whose RNA product is expressed in all tissues measured, but most abundantly in brain. Among a series of additional, unrelated subjects referred for clinical diagnostic testing who showed CNV affecting this locus, we identified four with exon-crossing deletions in association with neurodevelopmental abnormalities. No disruption of the LINC00299 coding sequence was seen in almost 14,000 control subjects. Together, these subjects with disruption of LINC00299 implicate this particular noncoding RNA in brain development and raise the possibility that, as a class, abnormalities of lincRNAs may play a significant role in human developmental disorders.
Journal: American Journal Of Human Genetics, 2012 Dec 7 DOI: 10.1016/j.ajhg.2012.10.016 PMID: 23217328.0 PMCID: PMC3516594
81. Linking DNA methyltransferases to epigenetic marks and nucleosome structure genome-wide in human tumor cells.
Jin, Ernst, Tiedemann, Xu, Sureshchandra, Kellis, Dalton, Liu, Choi, Robertson
DNA methylation, mediated by the combined action of three DNA methyltransferases (DNMT1, DNMT3A, and DNMT3B), is essential for mammalian development and is a major contributor to cellular transformation. To elucidate how DNA methylation is targeted, we mapped the genome-wide localization of all DNMTs and methylation, and examined the relationships among these markers, histone modifications, and nucleosome structure in a pluripotent human tumor cell line in its undifferentiated and differentiated states. Our findings reveal a strong link between DNMTs and transcribed loci, and that DNA methylation is not a simple sum of DNMT localization patterns. A comparison of the epigenomes of normal and cancerous stem cells, and pluripotent and differentiated states shows that the presence of at least two DNMTs is strongly associated with loci targeted for DNA hypermethylation. Taken together, these results shed important light on the determinants of DNA methylation and how it may become disrupted in cancer cells.
Journal: Cell Reports, 2012 Nov 29 DOI: 10.1016/j.celrep.2012.10.017 PMID: 23177624.0 PMCID: PMC3625945
80. Interpreting noncoding genetic variation in complex traits and human disease.
Ward, Kellis
Association studies provide genome-wide information about the genetic basis of complex disease, but medical research has focused primarily on protein-coding variants, owing to the difficulty of interpreting noncoding mutations. This picture has changed with advances in the systematic annotation of functional noncoding elements. Evolutionary conservation, functional genomics, chromatin state, sequence motifs and molecular quantitative trait loci all provide complementary information about the function of noncoding sequences. These functional maps can help with prioritizing variants on risk haplotypes, filtering mutations encountered in the clinic and performing systems-level analyses to reveal processes underlying disease associations. Advances in predictive modeling can enable data-set integration to reveal pathways shared across loci and alleles, and richer regulatory models can guide the search for epistatic interactions. Lastly, new massively parallel reporter experiments can systematically validate regulatory predictions. Ultimately, advances in regulatory and systems genomics can help unleash the value of whole-genome sequencing for personalized genomic risk assessment, diagnosis and treatment.
Journal: Nature Biotechnology, 2012 Nov DOI: 10.1038/nbt.2422 PMID: 23138309.0 PMCID: PMC3703467
79. Computational analysis of noncoding RNAs.
Washietl, Will, Hendrix, Goff, Rinn, Berger, Kellis
Noncoding RNAs have emerged as important key players in the cell. Understanding their surprisingly diverse range of functions is challenging for experimental and computational biology. Here, we review computational methods to analyze noncoding RNAs. The topics covered include basic and advanced techniques to predict RNA structures, annotation of noncoding RNAs in genomic data, mining RNA-seq data for novel transcripts and prediction of transcript structures, computational aspects of microRNAs, and database resources.
Journal: Wiley Interdisciplinary Reviews. Rna, 2012 Nov-Dec DOI: 10.1002/wrna.1134 PMID: 22991327.0 PMCID: PMC3472101
78. Analysis of variation at transcription factor binding sites in Drosophila and humans.
Spivakov, Akhtar, Kheradpour, Beal, Girardot, Koscielny, Herrero, Kellis, Furlong, Birney
Advances in sequencing technology have boosted population genomics and made it possible to map the positions of transcription factor binding sites (TFBSs) with high precision. Here we investigate TFBS variability by combining transcription factor binding maps generated by ENCODE, modENCODE, our previously published data and other sources with genomic variation data for human individuals and Drosophila isogenic lines. RESULTS: We introduce a metric of TFBS variability that takes into account changes in motif match associated with mutation and makes it possible to investigate TFBS functional constraints instance-by-instance as well as in sets that share common biological properties. We also take advantage of the emerging per-individual transcription factor binding data to show evidence that TFBS mutations, particularly at evolutionarily conserved sites, can be efficiently buffered to ensure coherent levels of transcription factor binding. CONCLUSIONS: Our analyses provide insights into the relationship between individual and interspecies variation and show evidence for the functional buffering of TFBS mutations in both humans and flies. In a broad perspective, these results demonstrate the potential of combining functional genomics and population genetics approaches for understanding gene regulation.
Journal: Genome Biology, 2012 Sep 28 DOI: 10.1186/gb-2012-13-9-r49 PMID: 22950968.0 PMCID: PMC3491393
77. Evidence of abundant purifying selection in humans for recently acquired regulatory functions.
Ward, Kellis
Although only 5% of the human genome is conserved across mammals, a substantially larger portion is biochemically active, raising the question of whether the additional elements evolve neutrally or confer a lineage-specific fitness advantage. To address this question, we integrate human variation information from the 1000 Genomes Project and activity data from the ENCODE Project. A broad range of transcribed and regulatory nonconserved elements show decreased human diversity, suggesting lineage-specific purifying selection. Conversely, conserved elements lacking activity show increased human diversity, suggesting that some recently became nonfunctional. Regulatory elements under human constraint in nonconserved regions were found near color vision and nerve-growth genes, consistent with purifying selection for recently evolved functions. Our results suggest continued turnover in regulatory regions, with at least an additional 4% of the human genome subject to lineage-specific constraint.
Journal: Science, 2012 Sep 28 DOI: 10.1126/science.1225057 PMID: 22956687.0 PMCID: PMC4104271
76. High depth, whole-genome sequencing of cholera isolates from Haiti and the Dominican Republic.
Sealfon, Gire, Ellis, Calderwood, Qadri, Hensley, Kellis, Ryan, LaRocque, Harris, Sabeti
Whole-genome sequencing is an important tool for understanding microbial evolution and identifying the emergence of functionally important variants over the course of epidemics. In October 2010, a severe cholera epidemic began in Haiti, with additional cases identified in the neighboring Dominican Republic. We used whole-genome approaches to sequence four Vibrio cholerae isolates from Haiti and the Dominican Republic and three additional V. cholerae isolates to a high depth of coverage (>2000x); four of the seven isolates were previously sequenced. RESULTS: Using these sequence data, we examined the effect of depth of coverage and sequencing platform on genome assembly and identification of sequence variants. We found that 50x coverage is sufficient to construct a whole-genome assembly and to accurately call most variants from 100 base pair paired-end sequencing reads. Phylogenetic analysis between the newly sequenced and thirty-three previously sequenced V. cholerae isolates indicates that the Haitian and Dominican Republic isolates are closest to strains from South Asia. The Haitian and Dominican Republic isolates form a tight cluster, with only four variants unique to individual isolates. These variants are located in the CTX region, the SXT region, and the core genome. Of the 126 mutations identified that separate the Haiti-Dominican Republic cluster from the V. cholerae reference strain (N16961), 73 are non-synonymous changes, and a number of these changes cluster in specific genes and pathways. CONCLUSIONS: Sequence variant analyses of V. cholerae isolates, including multiple isolates from the Haitian outbreak, identify coverage-specific and technology-specific effects on variant detection, and provide insight into genomic change and functional evolution during an epidemic.
Journal: Bmc Genomics, 2012 Sep 11 DOI: 10.1186/1471-2164-13-468 PMID: 22963323.0 PMCID: PMC3473251
75. An integrated encyclopedia of DNA elements in the human genome.
The ENCODE Project Consortium
The human genome encodes the blueprint of life, but the function of the vast majority of its nearly three billion bases is unknown. The Encyclopedia of DNA Elements (ENCODE) project has systematically mapped regions of transcription, transcription factor association, chromatin structure and histone modification. These data enabled us to assign biochemical functions for 80% of the genome, in particular outside of the well-studied protein-coding regions. Many discovered candidate regulatory elements are physically associated with one another and with expressed genes, providing new insights into the mechanisms of gene regulation. The newly identified elements also show a statistical correspondence to sequence variants linked to human disease, and can thereby guide interpretation of this variation. Overall, the project provides new insights into the organization and regulation of our genes and genome, and is an expansive resource of functional annotations for biomedical research.
Journal: Nature, 2012 Sep 6 DOI: 10.1038/nature11247 PMID: 22955616.0 PMCID: PMC3439153
74. GENCODE: the reference human genome annotation for The ENCODE Project.
Harrow, Frankish, Gonzalez, Tapanari, Diekhans, Kokocinski, Aken, Barrell, Zadissa, Searle, Barnes, Bignell, Boychenko, Hunt, Kay, Mukherjee, Rajan, Despacio-Reyes, Saunders, Steward, Harte, Lin, Howald, Tanzer, Derrien, Chrast, Walters, Balasubramanian, Pei, Tress, Rodriguez, Ezkurdia, van, Brent, Haussler, Kellis, Valencia, Reymond, Gerstein, Guigo, Hubbard
The GENCODE Consortium aims to identify all gene features in the human genome using a combination of computational analysis, manual annotation, and experimental validation. Since the first public release of this annotation data set, few new protein-coding loci have been added, yet the number of alternative splicing transcripts annotated has steadily increased. The GENCODE 7 release contains 20,687 protein-coding and 9640 long noncoding RNA loci and has 33,977 coding transcripts not represented in UCSC genes and RefSeq. It also has the most comprehensive annotation of long noncoding RNA (lncRNA) loci publicly available with the predominant transcript form consisting of two exons. We have examined the completeness of the transcript annotation and found that 35% of transcriptional start sites are supported by CAGE clusters and 62% of protein-coding genes have annotated polyA sites. Over one-third of GENCODE protein-coding genes are supported by peptide hits derived from mass spectrometry spectra submitted to Peptide Atlas. New models derived from the Illumina Body Map 2.0 RNA-seq data identify 3689 new loci not currently in GENCODE, of which 3127 consist of two exon models indicating that they are possibly unannotated long noncoding loci. GENCODE 7 is publicly available from gencodegenes.org and via the Ensembl and UCSC Genome Browsers.
Journal: Genome Research, 2012 Sep DOI: 10.1101/gr.135350.111 PMID: 22955987.0 PMCID: PMC3431492
73. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia.
Landt, Marinov, Kundaje, Kheradpour, Pauli, Batzoglou, Bernstein, Bickel, Brown, Cayting, Chen, DeSalvo, Epstein, Fisher-Aylor, Euskirchen, Gerstein, Gertz, Hartemink, Hoffman, Iyer, Jung, Karmakar, Kellis, Kharchenko, Li, Liu, Liu, Ma, Milosavljevic, Myers, Park, Pazin, Perry, Raha, Reddy, Rozowsky, Shoresh, Sidow, Slattery, Stamatoyannopoulos, Tolstorukov, White, Xi, Farnham, Lieb, Wold, Snyder
Chromatin immunoprecipitation (ChIP) followed by high-throughput DNA sequencing (ChIP-seq) has become a valuable and widely used approach for mapping the genomic location of transcription-factor binding and histone modifications in living cells. Despite its widespread use, there are considerable differences in how these experiments are conducted, how the results are scored and evaluated for quality, and how the data and metadata are archived for public use. These practices affect the quality and utility of any global ChIP experiment. Through our experience in performing ChIP-seq experiments, the ENCODE and modENCODE consortia have developed a set of working standards and guidelines for ChIP experiments that are updated routinely. The current guidelines address antibody validation, experimental replication, sequencing depth, data and metadata reporting, and data quality assessment. We discuss how ChIP quality, assessed in these ways, affects different uses of ChIP-seq data. All data sets used in the analysis have been deposited for public viewing and downloading at the ENCODE (http://encodeproject.org/ENCODE/) and modENCODE (http://www.modencode.org/) portals.
Journal: Genome Research, 2012 Sep DOI: 10.1101/gr.136184.111 PMID: 22955991.0 PMCID: PMC3431496
72. Wisdom of crowds for robust gene network inference.
Marbach, Costello, Kuffner, Vega, Prill, Camacho, Allison, Kellis, Collins, Stolovitzky
Reconstructing gene regulatory networks from high-throughput data is a long-standing challenge. Through the Dialogue on Reverse Engineering Assessment and Methods (DREAM) project, we performed a comprehensive blind assessment of over 30 network inference methods on Escherichia coli, Staphylococcus aureus, Saccharomyces cerevisiae and in silico microarray data. We characterize the performance, data requirements and inherent biases of different inference approaches, and we provide guidelines for algorithm application and development. We observed that no single inference method performs optimally across all data sets. In contrast, integration of predictions from multiple inference methods shows robust and high performance across diverse data sets. We thereby constructed high-confidence networks for E. coli and S. aureus, each comprising ~1,700 transcriptional interactions at a precision of ~50%. We experimentally tested 53 previously unobserved regulatory interactions in E. coli, of which 23 (43%) were supported. Our results establish community-based methods as a powerful and robust tool for the inference of transcriptional gene regulatory networks.
Journal: Nature Methods, 2012 Jul 15 DOI: 10.1038/nmeth.2016 PMID: 22796662.0 PMCID: PMC3512113
71. Predictive regulatory models in Drosophila melanogaster by integrative inference of transcriptional networks.
Marbach, Roy, Ay, Meyer, Candeias, Kahveci, Bristow, Kellis
Gaining insights on gene regulation from large-scale functional data sets is a grand challenge in systems biology. In this article, we develop and apply methods for transcriptional regulatory network inference from diverse functional genomics data sets and demonstrate their value for gene function and gene expression prediction. We formulate the network inference problem in a machine-learning framework and use both supervised and unsupervised methods to predict regulatory edges by integrating transcription factor (TF) binding, evolutionarily conserved sequence motifs, gene expression, and chromatin modification data sets as input features. Applying these methods to Drosophila melanogaster, we predict approximately 300,000 regulatory edges in a network of approximately 600 TFs and 12,000 target genes. We validate our predictions using known regulatory interactions, gene functional annotations, tissue-specific expression, protein-protein interactions, and three-dimensional maps of chromosome conformation. We use the inferred network to identify putative functions for hundreds of previously uncharacterized genes, including many in nervous system development, which are independently confirmed based on their tissue-specific expression patterns. Last, we use the regulatory network to predict target gene expression levels as a function of TF expression, and find significantly higher predictive power for integrative networks than for motif or ChIP-based networks. Our work reveals the complementarity between physical evidence of regulatory interactions (TF binding, motif conservation) and functional evidence (coordinated expression or chromatin patterns) and demonstrates the power of data integration for network inference and studies of gene regulation at the systems level.
Journal: Genome Research, 2012 Jul DOI: 10.1101/gr.127191.111 PMID: 22456606.0 PMCID: PMC3396374
70. Efficient algorithms for the reconciliation problem with gene duplication, horizontal transfer and loss.
Bansal, Alm, Kellis
Gene family evolution is driven by evolutionary events such as speciation, gene duplication, horizontal gene transfer and gene loss, and inferring these events in the evolutionary history of a given gene family is a fundamental problem in comparative and evolutionary genomics with numerous important applications. Solving this problem requires the use of a reconciliation framework, where the input consists of a gene family phylogeny and the corresponding species phylogeny, and the goal is to reconcile the two by postulating speciation, gene duplication, horizontal gene transfer and gene loss events. This reconciliation problem is referred to as duplication-transfer-loss (DTL) reconciliation and has been extensively studied in the literature. Yet, even the fastest existing algorithms for DTL reconciliation are too slow for reconciling large gene families and for use in more sophisticated applications such as gene tree or species tree reconstruction. RESULTS: We present two new algorithms for the DTL reconciliation problem that are dramatically faster than existing algorithms, both asymptotically and in practice. We also extend the standard DTL reconciliation model by considering distance-dependent transfer costs, which allow for more accurate reconciliation and give an efficient algorithm for DTL reconciliation under this extended model. We implemented our new algorithms and demonstrated up to 100 000-fold speed-up over existing methods, using both simulated and biological datasets. This dramatic improvement makes it possible to use DTL reconciliation for performing rigorous evolutionary analyses of large gene families and enables its use in advanced reconciliation-based gene and species tree reconstruction methods. AVAILABILITY: Our programs can be freely downloaded from http://compbio.mit.edu/ranger-dtl/.
Journal: Bioinformatics, 2012 Jun 15 DOI: 10.1093/bioinformatics/bts225 PMID: 22689773.0 PMCID: PMC3371857
69. RNA folding with soft constraints: reconciliation of probing data and thermodynamic secondary structure prediction.
Washietl, Hofacker, Stadler, Kellis
Thermodynamic folding algorithms and structure probing experiments are commonly used to determine the secondary structure of RNAs. Here we propose a formal framework to reconcile information from both prediction algorithms and probing experiments. The thermodynamic energy parameters are adjusted using 'pseudo-energies' to minimize the discrepancy between prediction and experiment. Our framework differs from related approaches that used pseudo-energies in several key aspects. (i) The energy model is only changed when necessary and no adjustments are made if prediction and experiment are consistent. (ii) Pseudo-energies remain biophysically interpretable and hold positional information where experiment and model disagree. (iii) The whole thermodynamic ensemble of structures is considered thus allowing to reconstruct mixtures of suboptimal structures from seemingly contradicting data. (iv) The noise of the energy model and the experimental data is explicitly modeled leading to an intuitive weighting factor through which the problem can be seen as folding with 'soft' constraints of different strength. We present an efficient algorithm to iteratively calculate pseudo-energies within this framework and demonstrate how this approach can be used in combination with SHAPE chemical probing data to improve secondary structure prediction. We further demonstrate that the pseudo-energies correlate with biophysical effects that are known to affect RNA folding such as chemical nucleotide modifications and protein binding.
Journal: Nucleic Acids Research, 2012 May DOI: 10.1093/nar/gks009 PMID: 22287623.0 PMCID: PMC3378861
68. Unified modeling of gene duplication, loss, and coalescence using a locus tree.
Rasmussen, Kellis
Gene phylogenies provide a rich source of information about the way evolution shapes genomes, populations, and phenotypes. In addition to substitutions, evolutionary events such as gene duplication and loss (as well as horizontal transfer) play a major role in gene evolution, and many phylogenetic models have been developed in order to reconstruct and study these events. However, these models typically make the simplifying assumption that population-related effects such as incomplete lineage sorting (ILS) are negligible. While this assumption may have been reasonable in some settings, it has become increasingly problematic as increased genome sequencing has led to denser phylogenies, where effects such as ILS are more prominent. To address this challenge, we present a new probabilistic model, DLCoal, that defines gene duplication and loss in a population setting, such that coalescence and ILS can be directly addressed. Interestingly, this model implies that in addition to the usual gene tree and species tree, there exists a third tree, the locus tree, which will likely have many applications. Using this model, we develop the first general reconciliation method that accurately infers gene duplications and losses in the presence of ILS, and we show its improved inference of orthologs, paralogs, duplications, and losses for a variety of clades, including flies, fungi, and primates. Also, our simulations show that gene duplications increase the frequency of ILS, further illustrating the importance of a joint model. Going forward, we believe that this unified model can offer insights to questions in both phylogenetics and population genetics.
Journal: Genome Research, 2012 Apr DOI: 10.1101/gr.123901.111 PMID: 22271778.0 PMCID: PMC3317157
67. ChromHMM: automating chromatin-state discovery and characterization.
Ernst, Kellis
Chromatin-state annotation using combinations of chromatin modification patterns has emerged as a powerful approach for discovering regulatory regions and their cell type-specific activity patterns and for interpreting disease-association studies. However, the computational challenge of learning chromatin-state models from large numbers of chromatin modi- fication datasets in multiple cell types still requires extensive bio- informatics expertise. To address this challenge, we developed ChromHMM, an automated computational system for learning chromatin states, characterizing their biological functions and correlations with large-scale functional datasets and visualizing the resulting genome-wide maps of chromatin-state annotations. ChromHMM is based on a multivariate hidden Markov model that models the observed combination of chromatin marks using a product of independent Bernoulli random variables2, which enables robust learning of complex patterns of many chromatin modifications. As input, it receives a list of aligned reads for each chromatin mark, which are automatically converted into presence or absence calls for each mark across the genome, based on a Poisson background distribution. One can use an optional additional input of aligned reads for a control dataset to either adjust the threshold for present or absent calls, or as an additional input mark. Alternatively, the user can input files that contain calls from an independent peak caller. By default, chromatin states are analyzed at 200-base-pair intervals that roughly approximate nucleosome sizes, but smaller or larger windows can be specified. We also developed an improved parameter-initialization procedure that enables relatively efficient inference of comparable models across different numbers of states.
Journal: Nature Methods, 2012 Feb 28 DOI: 10.1038/nmeth.1906 PMID: 22373907.0 PMCID: PMC3577932
66. Systematic dissection and optimization of inducible enhancers in human cells using a massively parallel reporter assay.
Melnikov, Murugan, Zhang, Tesileanu, Wang, Rogov, Feizi, Gnirke, Callan, Kinney, Kellis, Lander, Mikkelsen
Learning to read and write the transcriptional regulatory code is of central importance to progress in genetic analysis and engineering. Here we describe a massively parallel reporter assay (MPRA) that facilitates the systematic dissection of transcriptional regulatory elements. In MPRA, microarray-synthesized DNA regulatory elements and unique sequence tags are cloned into plasmids to generate a library of reporter constructs. These constructs are transfected into cells and tag expression is assayed by high-throughput sequencing. We apply MPRA to compare >27,000 variants of two inducible enhancers in human cells: a synthetic cAMP-regulated enhancer and the virus-inducible interferon-beta enhancer. We first show that the resulting data define accurate maps of functional transcription factor binding sites in both enhancers at single-nucleotide resolution. We then use the data to train quantitative sequence-activity models (QSAMs) of the two enhancers. We show that QSAMs from two cellular states can be combined to design enhancer variants that optimize potentially conflicting objectives, such as maximizing induced activity while minimizing basal activity.
Journal: Nature Biotechnology, 2012 Feb 26 DOI: 10.1038/nbt.2137 PMID: 22371084.0 PMCID: PMC3297981
65. Evolution at the subgene level: domain rearrangements in the Drosophila phylogeny.
Wu, Rasmussen, Kellis
Although the possibility of gene evolution by domain rearrangements has long been appreciated, current methods for reconstructing and systematically analyzing gene family evolution are limited to events such as duplication, loss, and sometimes, horizontal transfer. However, within the Drosophila clade, we find domain rearrangements occur in 35.9% of gene families, and thus, any comprehensive study of gene evolution in these species will need to account for such events. Here, we present a new computational model and algorithm for reconstructing gene evolution at the domain level. We develop a method for detecting homologous domains between genes and present a phylogenetic algorithm for reconstructing maximum parsimony evolutionary histories that include domain generation, duplication, loss, merge (fusion), and split (fission) events. Using this method, we find that genes involved in fusion and fission are enriched in signaling and development, suggesting that domain rearrangements and reuse may be crucial in these processes. We also find that fusion is more abundant than fission, and that fusion and fission events occur predominantly alongside duplication, with 92.5% and 34.3% of fusion and fission events retaining ancestral architectures in the duplicated copies. We provide a catalog of approximately 9,000 genes that undergo domain rearrangement across nine sequenced species, along with possible mechanisms for their formation. These results dramatically expand on evolution at the subgene level and offer several insights into how new genes and functions arise between species.
Journal: Molecular Biology And Evolution, 2012 Feb DOI: 10.1093/molbev/msr222 PMID: 21900599.0 PMCID: PMC3258039
64. Preface: RECOMB Systems Biology, Regulatory Genomics, and DREAM 2011 special issue.
Califano, Kellis, Stolovitzky
Journal: Journal Of Computational Biology, 2012 Feb DOI: 10.1089/cmb.2012.010p PMID: 22300312.0
63. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants.
Ward, Kellis
The resolution of genome-wide association studies (GWAS) is limited by the linkage disequilibrium (LD) structure of the population being studied. Selecting the most likely causal variants within an LD block is relatively straightforward within coding sequence, but is more difficult when all variants are intergenic. Predicting functional non-coding sequence has been recently facilitated by the availability of conservation and epigenomic information. We present HaploReg, a tool for exploring annotations of the non-coding genome among the results of published GWAS or novel sets of variants. Using LD information from the 1000 Genomes Project, linked SNPs and small indels can be visualized along with their predicted chromatin state in nine cell types, conservation across mammals and their effect on regulatory motifs. Sets of SNPs, such as those resulting from GWAS, are analyzed for an enrichment of cell type-specific enhancers. HaploReg will be useful to researchers developing mechanistic hypotheses of the impact of non-coding variants on clinical phenotypes and normal variation. The HaploReg database is available at http://compbio.mit.edu/HaploReg.
Journal: Nucleic Acids Research, 2012 Jan DOI: 10.1093/nar/gkr917 PMID: 22064851.0 PMCID: PMC3245002
62. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma.
Wiggs, Yaspan, Hauser, Kang, Allingham, Olson, Abdrabou, Fan, Wang, Brodeur, Budenz, Caprioli, Crenshaw, Crooks, Delbono, Doheny, Friedman, Gaasterland, Gaasterland, Laurie, Lee, Lichter, Loomis, Liu, Medeiros, McCarty, Mirel, Moroi, Musch, Realini, Rozsa, Schuman, Scott, Singh, Stein, Trager, Vanveldhuisen, Vollrath, Wollstein, Yoneyama, Zhang, Weinreb, Ernst, Kellis, Masuda, Zack, Richards, Pericak-Vance, Pasquale, Haines
Optic nerve degeneration caused by glaucoma is a leading cause of blindness worldwide. Patients affected by the normal-pressure form of glaucoma are more likely to harbor risk alleles for glaucoma-related optic nerve disease. We have performed a meta-analysis of two independent genome-wide association studies for primary open angle glaucoma (POAG) followed by a normal-pressure glaucoma (NPG, defined by intraocular pressure (IOP) less than 22 mmHg) subgroup analysis. The single-nucleotide polymorphisms that showed the most significant associations were tested for association with a second form of glaucoma, exfoliation-syndrome glaucoma. The overall meta-analysis of the GLAUGEN and NEIGHBOR dataset results (3,146 cases and 3,487 controls) identified significant associations between two loci and POAG: the CDKN2BAS region on 9p21 (rs2157719 [G], OR = 0.69 [95%CI 0.63-0.75], p = 1.86x10(-)(1)(8)), and the SIX1/SIX6 region on chromosome 14q23 (rs10483727 [A], OR = 1.32 [95%CI 1.21-1.43], p = 3.87x10(-)(1)(1)). In sub-group analysis two loci were significantly associated with NPG: 9p21 containing the CDKN2BAS gene (rs2157719 [G], OR = 0.58 [95% CI 0.50-0.67], p = 1.17x10(-)(1)(2)) and a probable regulatory region on 8q22 (rs284489 [G], OR = 0.62 [95% CI 0.53-0.72], p = 8.88x10(-)(1)(0)). Both NPG loci were also nominally associated with a second type of glaucoma, exfoliation syndrome glaucoma (rs2157719 [G], OR = 0.59 [95% CI 0.41-0.87], p = 0.004 and rs284489 [G], OR = 0.76 [95% CI 0.54-1.06], p = 0.021), suggesting that these loci might contribute more generally to optic nerve degeneration in glaucoma. Because both loci influence transforming growth factor beta (TGF-beta) signaling, we performed a genomic pathway analysis that showed an association between the TGF-beta pathway and NPG (permuted p = 0.009). These results suggest that neuro-protective therapies targeting TGF-beta signaling could be effective for multiple forms of glaucoma.
Journal: Plos Genetics, 2012 DOI: 10.1371/journal.pgen.1002654 PMID: 22570617.0 PMCID: PMC3343074
61. Combinatorial patterning of chromatin regulators uncovered by genome-wide location analysis in human cells.
Ram, Goren, Amit, Shoresh, Yosef, Ernst, Kellis, Gymrek, Issner, Coyne, Durham, Zhang, Donaghey, Epstein, Regev, Bernstein
Hundreds of chromatin regulators (CRs) control chromatin structure and function by catalyzing and binding histone modifications, yet the rules governing these key processes remain obscure. Here, we present a systematic approach to infer CR function. We developed ChIP-string, a meso-scale assay that combines chromatin immunoprecipitation with a signature readout of 487 representative loci. We applied ChIP-string to screen 145 antibodies, thereby identifying effective reagents, which we used to map the genome-wide binding of 29 CRs in two cell types. We found that specific combinations of CRs colocalize in characteristic patterns at distinct chromatin environments, at genes of coherent functions, and at distal regulatory elements. When comparing between cell types, CRs redistribute to different loci but maintain their modular and combinatorial associations. Our work provides a multiplex method that substantially enhances the ability to monitor CR binding, presents a large resource of CR maps, and reveals common principles for combinatorial CR function.
Journal: Cell, 2011 Dec 23 DOI: 10.1016/j.cell.2011.09.057 PMID: 22196736.0 PMCID: PMC3312319
60. Evidence of abundant stop codon readthrough in Drosophila and other metazoa.
Jungreis, Lin, Spokony, Chan, Negre, Victorsen, White, Kellis
While translational stop codon readthrough is often used by viral genomes, it has been observed for only a handful of eukaryotic genes. We previously used comparative genomics evidence to recognize protein-coding regions in 12 species of Drosophila and showed that for 149 genes, the open reading frame following the stop codon has a protein-coding conservation signature, hinting that stop codon readthrough might be common in Drosophila. We return to this observation armed with deep RNA sequence data from the modENCODE project, an improved higher-resolution comparative genomics metric for detecting protein-coding regions, comparative sequence information from additional species, and directed experimental evidence. We report an expanded set of 283 readthrough candidates, including 16 double-readthrough candidates; these were manually curated to rule out alternatives such as A-to-I editing, alternative splicing, dicistronic translation, and selenocysteine incorporation. We report experimental evidence of translation using GFP tagging and mass spectrometry for several readthrough regions. We find that the set of readthrough candidates differs from other genes in length, composition, conservation, stop codon context, and in some cases, conserved stem-loops, providing clues about readthrough regulation and potential mechanisms. Lastly, we expand our studies beyond Drosophila and find evidence of abundant readthrough in several other insect species and one crustacean, and several readthrough candidates in nematode and human, suggesting that functionally important translational stop codon readthrough is significantly more prevalent in Metazoa than previously recognized.
Journal: Genome Research, 2011 Dec DOI: 10.1101/gr.119974.110 PMID: 21994247.0 PMCID: PMC3227100
59. Locating protein-coding sequences under selection for additional, overlapping functions in 29 mammalian genomes.
Lin, Kheradpour, Washietl, Parker, Pedersen, Kellis
The degeneracy of the genetic code allows protein-coding DNA and RNA sequences to simultaneously encode additional, overlapping functional elements. A sequence in which both protein-coding and additional overlapping functions have evolved under purifying selection should show increased evolutionary conservation compared to typical protein-coding genes--especially at synonymous sites. In this study, we use genome alignments of 29 placental mammals to systematically locate short regions within human ORFs that show conspicuously low estimated rates of synonymous substitution across these species. The 29-species alignment provides statistical power to locate more than 10,000 such regions with resolution down to nine-codon windows, which are found within more than a quarter of all human protein-coding genes and contain approximately 2% of their synonymous sites. We collect numerous lines of evidence that the observed synonymous constraint in these regions reflects selection on overlapping functional elements including splicing regulatory elements, dual-coding genes, RNA secondary structures, microRNA target sites, and developmental enhancers. Our results show that overlapping functional elements are common in mammalian genes, despite the vast genomic landscape.
Journal: Genome Research, 2011 Nov DOI: 10.1101/gr.108753.110 PMID: 21994248.0 PMCID: PMC3205576
58. New families of human regulatory RNA structures identified by comparative analysis of vertebrate genomes.
Parker, Moltke, Roth, Washietl, Wen, Kellis, Breaker, Pedersen
Regulatory RNA structures are often members of families with multiple paralogous instances across the genome. Family members share functional and structural properties, which allow them to be studied as a whole, facilitating both bioinformatic and experimental characterization. We have developed a comparative method, EvoFam, for genome-wide identification of families of regulatory RNA structures, based on primary sequence and secondary structure similarity. We apply EvoFam to a 41-way genomic vertebrate alignment. Genome-wide, we identify 220 human, high-confidence families outside protein-coding regions comprising 725 individual structures, including 48 families with known structural RNA elements. Known families identified include both noncoding RNAs, e.g., miRNAs and the recently identified MALAT1/MEN beta lincRNA family; and cis-regulatory structures, e.g., iron-responsive elements. We also identify tens of new families supported by strong evolutionary evidence and other statistical evidence, such as GO term enrichments. For some of these, detailed analysis has led to the formulation of specific functional hypotheses. Examples include two hypothesized auto-regulatory feedback mechanisms: one involving six long hairpins in the 3'-UTR of MAT2A, a key metabolic gene that produces the primary human methyl donor S-adenosylmethionine; the other involving a tRNA-like structure in the intron of the tRNA maturation gene POP1. We experimentally validate the predicted MAT2A structures. Finally, we identify potential new regulatory networks, including large families of short hairpins enriched in immunity-related genes, e.g., TNF, FOS, and CTLA4, which include known transcript destabilizing elements. Our findings exemplify the diversity of post-transcriptional regulation and provide a resource for further characterization of new regulatory mechanisms and families of noncoding RNAs.
Journal: Genome Research, 2011 Nov DOI: 10.1101/gr.112516.110 PMID: 21994249.0 PMCID: PMC3205577
57. A high-resolution map of human evolutionary constraint using 29 mammals.
Lindblad-Toh, Garber, Zuk, Lin, Parker, Washietl, Kheradpour, Ernst, Jordan, Mauceli, Ward, Lowe, Holloway, Clamp, Gnerre, Alfoldi, Beal, Chang, Clawson, Cuff, Di, Fitzgerald, Flicek, Guttman, Hubisz, Jaffe, Jungreis, Kent, Kostka, Lara, Martins, Massingham, Moltke, Raney, Rasmussen, Robinson, Stark, Vilella, Wen, Xie, Zody, Baldwin, Bloom, Chin, Heiman, Nicol, Nusbaum, Young, Wilkinson, Worley, Kovar, Muzny, Gibbs, Cree, Dihn, Fowler, Jhangiani, Joshi, Lee, Lewis, Nazareth, Okwuonu, Santibanez, Warren, Mardis, Weinstock, Wilson, Delehaunty, Dooling, Fronik, Fulton, Fulton, Graves, Minx, Sodergren, Birney, Margulies, Herrero, Green, Haussler, Siepel, Goldman, Pollard, Pedersen, Lander, Kellis
The comparison of related genomes has emerged as a powerful lens for genome interpretation. Here we report the sequencing and comparative analysis of 29 eutherian genomes. We confirm that at least 5.5% of the human genome has undergone purifying selection, and locate constrained elements covering approximately 4.2% of the genome. We use evolutionary signatures and comparisons with experimental data sets to suggest candidate functions for approximately 60% of constrained bases. These elements reveal a small number of new coding exons, candidate stop codon readthrough events and over 10,000 regions of overlapping synonymous constraint within protein-coding exons. We find 220 candidate RNA structural families, and nearly a million elements overlapping potential promoter, enhancer and insulator regions. We report specific amino acid residues that have undergone positive selection, 280,000 non-coding elements exapted from mobile elements and more than 1,000 primate- and human-accelerated elements. Overlap with disease-associated variants indicates that our findings will be relevant for studies of human biology, health and disease.
Journal: Nature, 2011 Oct 12 DOI: 10.1038/nature10530 PMID: 21993624.0 PMCID: PMC3207357
56. Dynamics of the epigenetic landscape during erythroid differentiation after GATA1 restoration.
Wu, Cheng, Keller, Ernst, Kumar, Mishra, Morrissey, Dorman, Chen, Drautz, Giardine, Shibata, Song, Pimkin, Crawford, Furey, Kellis, Miller, Taylor, Schuster, Zhang, Chiaromonte, Blobel, Weiss, Hardison
Interplays among lineage-specific nuclear proteins, chromatin modifying enzymes, and the basal transcription machinery govern cellular differentiation, but their dynamics of action and coordination with transcriptional control are not fully understood. Alterations in chromatin structure appear to establish a permissive state for gene activation at some loci, but they play an integral role in activation at other loci. To determine the predominant roles of chromatin states and factor occupancy in directing gene regulation during differentiation, we mapped chromatin accessibility, histone modifications, and nuclear factor occupancy genome-wide during mouse erythroid differentiation dependent on the master regulatory transcription factor GATA1. Notably, despite extensive changes in gene expression, the chromatin state profiles (proportions of a gene in a chromatin state dominated by activating or repressive histone modifications) and accessibility remain largely unchanged during GATA1-induced erythroid differentiation. In contrast, gene induction and repression are strongly associated with changes in patterns of transcription factor occupancy. Our results indicate that during erythroid differentiation, the broad features of chromatin states are established at the stage of lineage commitment, largely independently of GATA1. These determine permissiveness for expression, with subsequent induction or repression mediated by distinctive combinations of transcription factors.
Journal: Genome Research, 2011 Oct DOI: 10.1101/gr.125088.111 PMID: 21795386.0 PMCID: PMC3202283
55. Three periods of regulatory innovation during vertebrate evolution.
Lowe, Kellis, Siepel, Raney, Clamp, Salama, Kingsley, Lindblad-Toh, Haussler
The gain, loss, and modification of gene regulatory elements may underlie a substantial proportion of phenotypic changes on animal lineages. To investigate the gain of regulatory elements throughout vertebrate evolution, we identified genome-wide sets of putative regulatory regions for five vertebrates, including humans. These putative regulatory regions are conserved nonexonic elements (CNEEs), which are evolutionarily conserved yet do not overlap any coding or noncoding mature transcript. We then inferred the branch on which each CNEE came under selective constraint. Our analysis identified three extended periods in the evolution of gene regulatory elements. Early vertebrate evolution was characterized by regulatory gains near transcription factors and developmental genes, but this trend was replaced by innovations near extracellular signaling genes, and then innovations near posttranslational protein modifiers.
Journal: Science, 2011 Aug 19 DOI: 10.1126/science.1202702 PMID: 21852499.0 PMCID: PMC3511857
54. PhyloCSF: a comparative genomics method to distinguish protein coding and non-coding regions.
Lin, Jungreis, Kellis
As high-throughput transcriptome sequencing provides evidence for novel transcripts in many species, there is a renewed need for accurate methods to classify small genomic regions as protein coding or non-coding. We present PhyloCSF, a novel comparative genomics method that analyzes a multispecies nucleotide sequence alignment to determine whether it is likely to represent a conserved protein-coding region, based on a formal statistical comparison of phylogenetic codon models. RESULTS: We show that PhyloCSF's classification performance in 12-species Drosophila genome alignments exceeds all other methods we compared in a previous study. We anticipate that this method will be widely applicable as the transcriptomes of many additional species, tissues and subcellular compartments are sequenced, particularly in the context of ENCODE and modENCODE, and as interest grows in long non-coding RNAs, often initially recognized by their lack of protein coding potential rather than conserved RNA secondary structures. AVAILABILITY AND IMPLEMENTATION: The Objective Caml source code and executables for GNU/Linux and Mac OS X are freely available at http://compbio.mit.edu/PhyloCSF CONTACT: mlin@mit.edu; manoli@mit.edu.
Journal: Bioinformatics, 2011 Jul 1 DOI: 10.1093/bioinformatics/btr209 PMID: 21685081.0 PMCID: PMC3117341
53. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters.
Hung, Wang, Lin, Koegel, Kotake, Grant, Horlings, Shah, Umbricht, Wang, Wang, Kong, Langerod, Borresen-Dale, Kim, van, Sukumar, Whitfield, Kellis, Xiong, Wong, Chang
Transcription of long noncoding RNAs (lncRNAs) within gene regulatory elements can modulate gene activity in response to external stimuli, but the scope and functions of such activity are not known. Here we use an ultrahigh-density array that tiles the promoters of 56 cell-cycle genes to interrogate 108 samples representing diverse perturbations. We identify 216 transcribed regions that encode putative lncRNAs, many with RT-PCR-validated periodic expression during the cell cycle, show altered expression in human cancers and are regulated in expression by specific oncogenic stimuli, stem cell differentiation or DNA damage. DNA damage induces five lncRNAs from the CDKN1A promoter, and one such lncRNA, named PANDA, is induced in a p53-dependent manner. PANDA interacts with the transcription factor NF-YA to limit expression of pro-apoptotic genes; PANDA depletion markedly sensitized human fibroblasts to apoptosis by doxorubicin. These findings suggest potentially widespread roles for promoter lncRNAs in cell-growth control.
Journal: Nature Genetics, 2011 Jun 5 DOI: 10.1038/ng.848 PMID: 21642992.0 PMCID: PMC3652667
52. Comparative functional genomics of the fission yeasts.
Rhind, Chen, Yassour, Thompson, Haas, Habib, Wapinski, Roy, Lin, Heiman, Young, Furuya, Guo, Pidoux, Chen, Robbertse, Goldberg, Aoki, Bayne, Berlin, Desjardins, Dobbs, Dukaj, Fan, FitzGerald, French, Gujja, Hansen, Keifenheim, Levin, Mosher, Muller, Pfiffner, Priest, Russ, Smialowska, Swoboda, Sykes, Vaughn, Vengrova, Yoder, Zeng, Allshire, Baulcombe, Birren, Brown, Ekwall, Kellis, Leatherwood, Levin, Margalit, Martienssen, Nieduszynski, Spatafora, Friedman, Dalgaard, Baumann, Niki, Regev, Nusbaum
The fission yeast clade--comprising Schizosaccharomyces pombe, S. octosporus, S. cryophilus, and S. japonicus--occupies the basal branch of Ascomycete fungi and is an important model of eukaryote biology. A comparative annotation of these genomes identified a near extinction of transposons and the associated innovation of transposon-free centromeres. Expression analysis established that meiotic genes are subject to antisense transcription during vegetative growth, which suggests a mechanism for their tight regulation. In addition, trans-acting regulators control new genes within the context of expanded functional modules for meiosis and stress response. Differences in gene content and regulation also explain why, unlike the budding yeast of Saccharomycotina, fission yeasts cannot use ethanol as a primary carbon source. These analyses elucidate the genome structure and gene regulation of fission yeast and provide tools for investigation across the Schizosaccharomyces clade.
Journal: Science, 2011 May 20 DOI: 10.1126/science.1203357 PMID: 21511999.0 PMCID: PMC3131103
51. An epigenetic signature for monoallelic olfactory receptor expression.
Magklara, Yen, Colquitt, Clowney, Allen, Markenscoff-Papadimitriou, Evans, Kheradpour, Mountoufaris, Carey, Barnea, Kellis, Lomvardas
Constitutive heterochromatin is traditionally viewed as the static form of heterochromatin that silences pericentromeric and telomeric repeats in a cell cycle- and differentiation-independent manner. Here, we show that, in the mouse olfactory epithelium, olfactory receptor (OR) genes are marked in a highly dynamic fashion with the molecular hallmarks of constitutive heterochromatin, H3K9me3 and H4K20me3. The cell type and developmentally dependent deposition of these marks along the OR clusters are, most likely, reversed during the process of OR choice to allow for monogenic and monoallelic OR expression. In contrast to the current view of OR choice, our data suggest that OR silencing takes place before OR expression, indicating that it is not the product of an OR-elicited feedback signal. Our findings suggest that chromatin-mediated silencing lays a molecular foundation upon which singular and stochastic selection for gene expression can be applied.
Journal: Cell, 2011 May 13 DOI: 10.1016/j.cell.2011.03.040 PMID: 21529909.0 PMCID: PMC3094500
50. Mapping and analysis of chromatin state dynamics in nine human cell types.
Ernst, Kheradpour, Mikkelsen, Shoresh, Ward, Epstein, Zhang, Wang, Issner, Coyne, Ku, Durham, Kellis, Bernstein
Chromatin profiling has emerged as a powerful means of genome annotation and detection of regulatory activity. The approach is especially well suited to the characterization of non-coding portions of the genome, which critically contribute to cellular phenotypes yet remain largely uncharted. Here we map nine chromatin marks across nine cell types to systematically characterize regulatory elements, their cell-type specificities and their functional interactions. Focusing on cell-type-specific patterns of promoters and enhancers, we define multicell activity profiles for chromatin state, gene expression, regulatory motif enrichment and regulator expression. We use correlations between these profiles to link enhancers to putative target genes, and predict the cell-type-specific activators and repressors that modulate them. The resulting annotations and regulatory predictions have implications for the interpretation of genome-wide association studies. Top-scoring disease single nucleotide polymorphisms are frequently positioned within enhancer elements specifically active in relevant cell types, and in some cases affect a motif instance for a predicted regulator, thus suggesting a mechanism for the association. Our study presents a general framework for deciphering cis-regulatory connections and their roles in disease.
Journal: Nature, 2011 May 5 DOI: 10.1038/nature09906 PMID: 21441907.0 PMCID: PMC3088773
49. A user's guide to the encyclopedia of DNA elements (ENCODE).
The ENCODE Project Consortium
The mission of the Encyclopedia of DNA Elements (ENCODE) Project is to enable the scientific and medical communities to interpret the human genome sequence and apply it to understand human biology and improve health. The ENCODE Consortium is integrating multiple technologies and approaches in a collective effort to discover and define the functional elements encoded in the human genome, including genes, transcripts, and transcriptional regulatory regions, together with their attendant chromatin states and DNA methylation patterns. In the process, standards to ensure high-quality data have been implemented, and novel algorithms have been developed to facilitate analysis. Data and derived results are made available through a freely accessible database. Here we provide an overview of the project and the resources it is generating and illustrate the application of ENCODE data to interpret the human genome.
Journal: Plos Biology, 2011 Apr DOI: 10.1371/journal.pbio.1001046 PMID: 21526222.0 PMCID: PMC3079585
48. Comprehensive analysis of the chromatin landscape in Drosophila melanogaster.
Kharchenko, Alekseyenko, Schwartz, Minoda, Riddle, Ernst, Sabo, Larschan, Gorchakov, Gu, Linder-Basso, Plachetka, Shanower, Tolstorukov, Luquette, Xi, Jung, Park, Bishop, Canfield, Sandstrom, Thurman, MacAlpine, Stamatoyannopoulos, Kellis, Elgin, Kuroda, Pirrotta, Karpen, Park
Chromatin is composed of DNA and a variety of modified histones and non-histone proteins, which have an impact on cell differentiation, gene regulation and other key cellular processes. Here we present a genome-wide chromatin landscape for Drosophila melanogaster based on eighteen histone modifications, summarized by nine prevalent combinatorial patterns. Integrative analysis with other data (non-histone chromatin proteins, DNase I hypersensitivity, GRO-Seq reads produced by engaged polymerase, short/long RNA products) reveals discrete characteristics of chromosomes, genes, regulatory elements and other functional domains. We find that active genes display distinct chromatin signatures that are correlated with disparate gene lengths, exon patterns, regulatory functions and genomic contexts. We also demonstrate a diversity of signatures among Polycomb targets that include a subset with paused polymerase. This systematic profiling and integrative analysis of chromatin signatures provides insights into how genomic elements are regulated, and will serve as a resource for future experimental investigations of genome structure and function.
Journal: Nature, 2011 Mar 24 DOI: 10.1038/nature09725 PMID: 21179089.0 PMCID: PMC3109908
47. A cis-regulatory map of the Drosophila genome.
Negre, Brown, Ma, Bristow, Miller, Wagner, Kheradpour, Eaton, Loriaux, Sealfon, Li, Ishii, Spokony, Chen, Hwang, Cheng, Auburn, Davis, Domanus, Shah, Morrison, Zieba, Suchy, Senderowicz, Victorsen, Bild, Grundstad, Hanley, MacAlpine, Mannervik, Venken, Bellen, White, Gerstein, Russell, Grossman, Ren, Posakony, Kellis, White
Systematic annotation of gene regulatory elements is a major challenge in genome science. Direct mapping of chromatin modification marks and transcriptional factor binding sites genome-wide has successfully identified specific subtypes of regulatory elements. In Drosophila several pioneering studies have provided genome-wide identification of Polycomb response elements, chromatin states, transcription factor binding sites, RNA polymerase II regulation and insulator elements; however, comprehensive annotation of the regulatory genome remains a significant challenge. Here we describe results from the modENCODE cis-regulatory annotation project. We produced a map of the Drosophila melanogaster regulatory genome on the basis of more than 300 chromatin immunoprecipitation data sets for eight chromatin features, five histone deacetylases and thirty-eight site-specific transcription factors at different stages of development. Using these data we inferred more than 20,000 candidate regulatory elements and validated a subset of predictions for promoters, enhancers and insulators in vivo. We identified also nearly 2,000 genomic regions of dense transcription factor binding associated with chromatin activity and accessibility. We discovered hundreds of new transcription factor co-binding relationships and defined a transcription factor network with over 800 potential regulatory relationships.
Journal: Nature, 2011 Mar 24 DOI: 10.1038/nature09990 PMID: 21430782.0 PMCID: PMC3179250
46. SubMAP: aligning metabolic pathways with subnetwork mappings.
Ay, Kellis, Kahveci
We consider the problem of aligning two metabolic pathways. Unlike traditional approaches, we do not restrict the alignment to one-to-one mappings between the molecules (nodes) of the input pathways (graphs). We follow the observation that, in nature, different organisms can perform the same or similar functions through different sets of reactions and molecules. The number and the topology of the molecules in these alternative sets often vary from one organism to another. With the motivation that an accurate biological alignment should be able to reveal these functionally similar molecule sets across different species, we develop an algorithm that first measures the similarities between different nodes using a mixture of homology and topological similarity. We combine the two metrics by employing an eigenvalue formulation. We then search for an alignment between the two input pathways that maximizes a similarity score, evaluated as the sum of the similarities of the mapped subnetworks of size at most a given integer k, and also does not contain any conflicting mappings. Here we prove that this maximization is NP-hard by a reduction from the maximum weight independent set (MWIS) problem. We then convert our problem to an instance of MWIS and use an efficient vertex-selection strategy to extract the mappings that constitute our alignment. We name our algorithm SubMAP (Subnetwork Mappings in Alignment of Pathways). We evaluate its accuracy and performance on real datasets. Our empirical results demonstrate that SubMAP can identify biologically relevant mappings that are missed by traditional alignment methods. Furthermore, we observe that SubMAP is scalable for metabolic pathways of arbitrary topology, including searching for a query pathway of size 70 against the complete KEGG database of 1,842 pathways. Implementation in C++ is available at http://bioinformatics.cise.ufl.edu/SubMAP.html.
Journal: Journal Of Computational Biology, 2011 Mar DOI: 10.1089/cmb.2010.0280 PMID: 21385030.0 PMCID: PMC3123932
45. Error and error mitigation in low-coverage genome assemblies.
Hubisz, Lin, Kellis, Siepel
The recent release of twenty-two new genome sequences has dramatically increased the data available for mammalian comparative genomics, but twenty of these new sequences are currently limited to approximately 2x coverage. Here we examine the extent of sequencing error in these 2x assemblies, and its potential impact in downstream analyses. By comparing 2x assemblies with high-quality sequences from the ENCODE regions, we estimate the rate of sequencing error to be 1-4 errors per kilobase. While this error rate is fairly modest, sequencing error can still have surprising effects. For example, an apparent lineage-specific insertion in a coding region is more likely to reflect sequencing error than a true biological event, and the length distribution of coding indels is strongly distorted by error. We find that most errors are contributed by a small fraction of bases with low quality scores, in particular, by the ends of reads in regions of single-read coverage in the assembly. We explore several approaches for automatic sequencing error mitigation (SEM), making use of the localized nature of sequencing error, the fact that it is well predicted by quality scores, and information about errors that comes from comparisons across species. Our automatic methods for error mitigation cannot replace the need for additional sequencing, but they do allow substantial fractions of errors to be masked or eliminated at the cost of modest amounts of over-correction, and they can reduce the impact of error in downstream phylogenomic analyses. Our error-mitigated alignments are available for download.
Journal: Plos One, 2011 Feb 14 DOI: 10.1371/journal.pone.0017034 PMID: 21340033.0 PMCID: PMC3038916
44. Preface: RECOMB Conference on Systems Biology, Regulatory Genomics, and DREAM Challenges 2010 special issue.
Kellis, Califano, Bar-Joseph
Journal: Journal Of Computational Biology, 2011 Feb DOI: 10.1089/cmb.2010.005p PMID: 21314452.0
43. A Bayesian approach for fast and accurate gene tree reconstruction.
Rasmussen, Kellis
Recent sequencing and computing advances have enabled phylogenetic analyses to expand to both entire genomes and large clades, thus requiring more efficient and accurate methods designed specifically for the phylogenomic context. Here, we present SPIMAP, an efficient Bayesian method for reconstructing gene trees in the presence of a known species tree. We observe many improvements in reconstruction accuracy, achieved by modeling multiple aspects of evolution, including gene duplication and loss (DL) rates, speciation times, and correlated substitution rate variation across both species and loci. We have implemented and applied this method on two clades of fully sequenced species, 12 Drosophila and 16 fungal genomes as well as simulated phylogenies and find dramatic improvements in reconstruction accuracy as compared with the most popular existing methods, including those that take the species tree into account. We find that reconstruction inaccuracies of traditional phylogenetic methods overestimate the number of DL events by as much as 2-3-fold, whereas our method achieves significantly higher accuracy. We feel that the results and methods presented here will have many important implications for future investigations of gene evolution.
Journal: Molecular Biology And Evolution, 2011 Jan DOI: 10.1093/molbev/msq189 PMID: 20660489.0 PMCID: PMC3002250
42. Identification of functional elements and regulatory circuits by Drosophila modENCODE.
Roy, Ernst, Kharchenko, Kheradpour, Negre, Eaton, Landolin, Bristow, Ma, Lin, Washietl, Arshinoff, Ay, Meyer, Robine, Washington, Di, Berezikov, Brown, Candeias, Carlson, Carr, Jungreis, Marbach, Sealfon, Tolstorukov, Will, Alekseyenko, Artieri, Booth, Brooks, Dai, Davis, Duff, Feng, Gorchakov, Gu, Henikoff, Kapranov, Li, MacAlpine, Malone, Minoda, Nordman, Okamura, Perry, Powell, Riddle, Sakai, Samsonova, Sandler, Schwartz, Sher, Spokony, Sturgill, van, Wan, Yang, Yu, Feingold, Good, Guyer, Lowdon, Ahmad, Andrews, Berger, Brenner, Brent, Cherbas, Elgin, Gingeras, Grossman, Hoskins, Kaufman, Kent, Kuroda, Orr-Weaver, Perrimon, Pirrotta, Posakony, Ren, Russell, Cherbas, Graveley, Lewis, Micklem, Oliver, Park, Celniker, Henikoff, Karpen, Lai, MacAlpine, Stein, White, Kellis
To gain insight into how genomic information is translated into cellular and developmental programs, the Drosophila model organism Encyclopedia of DNA Elements (modENCODE) project is comprehensively mapping transcripts, histone modifications, chromosomal proteins, transcription factors, replication proteins and intermediates, and nucleosome properties across a developmental time course and in multiple cell lines. We have generated more than 700 data sets and discovered protein-coding, noncoding, RNA regulatory, replication, and chromatin elements, more than tripling the annotated portion of the Drosophila genome. Correlated activity patterns of these elements reveal a functional regulatory network, which predicts putative new functions for genes, reveals stage- and tissue-specific regulators, and enables gene-expression prediction. Our results provide a foundation for directed experimental and computational studies in Drosophila and related species and also a model for systematic data integration toward comprehensive genomic and functional annotation.
Journal: Science, 2010 Dec 24 DOI: 10.1126/science.1198374 PMID: 21177974.0 PMCID: PMC3192495
41. Optimization of parameters for coverage of low molecular weight proteins.
Muller, Kohajda, Findeiss, Stadler, Washietl, Kellis, von, Kalkhof
Proteins with molecular weights of <25 kDa are involved in major biological processes such as ribosome formation, stress adaption (e.g., temperature reduction) and cell cycle control. Despite their importance, the coverage of smaller proteins in standard proteome studies is rather sparse. Here we investigated biochemical and mass spectrometric parameters that influence coverage and validity of identification. The underrepresentation of low molecular weight (LMW) proteins may be attributed to the low numbers of proteolytic peptides formed by tryptic digestion as well as their tendency to be lost in protein separation and concentration/desalting procedures. In a systematic investigation of the LMW proteome of Escherichia coli, a total of 455 LMW proteins (27% of the 1672 listed in the SwissProt protein database) were identified, corresponding to a coverage of 62% of the known cytosolic LMW proteins. Of these proteins, 93 had not yet been functionally classified, and five had not previously been confirmed at the protein level. In this study, the influences of protein extraction (either urea or TFA), proteolytic digestion (solely, and the combined usage of trypsin and AspN as endoproteases) and protein separation (gel- or non-gel-based) were investigated. Compared to the standard procedure based solely on the use of urea lysis buffer, in-gel separation and tryptic digestion, the complementary use of TFA for extraction or endoprotease AspN for proteolysis permits the identification of an extra 72 (32%) and 51 proteins (23%), respectively. Regarding mass spectrometry analysis with an LTQ Orbitrap mass spectrometer, collision-induced fragmentation (CID and HCD) and electron transfer dissociation using the linear ion trap (IT) or the Orbitrap as the analyzer were compared. IT-CID was found to yield the best identification rate, whereas IT-ETD provided almost comparable results in terms of LMW proteome coverage. The high overlap between the proteins identified with IT-CID and IT-ETD allowed the validation of 75% of the identified proteins using this orthogonal fragmentation technique. Furthermore, a new approach to evaluating and improving the completeness of protein databases that utilizes the program RNAcode was introduced and examined.
Journal: Analytical And Bioanalytical Chemistry, 2010 Dec DOI: 10.1007/s00216-010-4093-x PMID: 20803007.0 PMCID: PMC2990009
40. The NIH Roadmap Epigenomics Mapping Consortium.
Bernstein, Stamatoyannopoulos, Costello, Ren, Milosavljevic, Meissner, Kellis, Marra, Beaudet, Ecker, Farnham, Hirst, Lander, Mikkelsen, Thomson
The NIH Roadmap Epigenomics Mapping Consortium aims to produce a public resource of epigenomic maps for stem cells and primary ex vivo tissues selected to represent the normal counterparts of tissues and organ systems frequently involved in human disease.
Journal: Nature Biotechnology, 2010 Oct DOI: 10.1038/nbt1010-1045 PMID: 20944595.0 PMCID: PMC3607281
39. Discovery and characterization of chromatin states for systematic annotation of the human genome.
Ernst, Kellis
A plethora of epigenetic modifications have been described in the human genome and shown to play diverse roles in gene regulation, cellular differentiation and the onset of disease. Although individual modifications have been linked to the activity levels of various genetic functional elements, their combinatorial patterns are still unresolved and their potential for systematic de novo genome annotation remains untapped. Here, we use a multivariate Hidden Markov Model to reveal 'chromatin states' in human T cells, based on recurrent and spatially coherent combinations of chromatin marks. We define 51 distinct chromatin states, including promoter-associated, transcription-associated, active intergenic, large-scale repressed and repeat-associated states. Each chromatin state shows specific enrichments in functional annotations, sequence motifs and specific experimentally observed characteristics, suggesting distinct biological roles. This approach provides a complementary functional annotation of the human genome that reveals the genome-wide locations of diverse classes of epigenetic function.
Journal: Nature Biotechnology, 2010 Aug DOI: 10.1038/nbt.1662 PMID: 20657582.0 PMCID: PMC2919626
38. A comprehensive map of insulator elements for the Drosophila genome.
Negre, Brown, Shah, Kheradpour, Morrison, Henikoff, Feng, Ahmad, Russell, White, Stein, Henikoff, Kellis, White
Insulators are DNA sequences that control the interactions among genomic regulatory elements and act as chromatin boundaries. A thorough understanding of their location and function is necessary to address the complexities of metazoan gene regulation. We studied by ChIP-chip the genome-wide binding sites of 6 insulator-associated proteins-dCTCF, CP190, BEAF-32, Su(Hw), Mod(mdg4), and GAF-to obtain the first comprehensive map of insulator elements in Drosophila embryos. We identify over 14,000 putative insulators, including all classically defined insulators. We find two major classes of insulators defined by dCTCF/CP190/BEAF-32 and Su(Hw), respectively. Distributional analyses of insulators revealed that particular sub-classes of insulator elements are excluded between cis-regulatory elements and their target promoters; divide differentially expressed, alternative, and divergent promoters; act as chromatin boundaries; are associated with chromosomal breakpoints among species; and are embedded within active chromatin domains. Together, these results provide a map demarcating the boundaries of gene regulatory units and a framework for understanding insulator function during the development and evolution of Drosophila.
Journal: Plos Genetics, 2010 Jan 15 DOI: 10.1371/journal.pgen.1000814 PMID: 20084099.0 PMCID: PMC2797089
37. The Tasmanian devil transcriptome reveals Schwann cell origins of a clonally transmissible cancer.
Murchison, Tovar, Hsu, Bender, Kheradpour, Rebbeck, Obendorf, Conlan, Bahlo, Blizzard, Pyecroft, Kreiss, Kellis, Stark, Harkins, Marshall, Woods, Hannon, Papenfuss
The Tasmanian devil, a marsupial carnivore, is endangered because of the emergence of a transmissible cancer known as devil facial tumor disease (DFTD). This fatal cancer is clonally derived and is an allograft transmitted between devils by biting. We performed a large-scale genetic analysis of DFTD with microsatellite genotyping, a mitochondrial genome analysis, and deep sequencing of the DFTD transcriptome and microRNAs. These studies confirm that DFTD is a monophyletic clonally transmissible tumor and suggest that the disease is of Schwann cell origin. On the basis of these results, we have generated a diagnostic marker for DFTD and identify a suite of genes relevant to DFTD pathology and transmission. We provide a genomic data set for the Tasmanian devil that is applicable to cancer diagnosis, disease evolution, and conservation biology.
Journal: Science, 2010 Jan 1 DOI: 10.1126/science.1180616 PMID: 20044575.0 PMCID: PMC2982769
36. Motif Discovery in Physiological Datasets: A Methodology for Inferring Predictive Elements.
Syed, Stultz, Kellis, Indyk, Guttag
In this article, we propose a methodology for identifying predictive physiological patterns in the absence of prior knowledge. We use the principle of conservation to identify activity that consistently precedes an outcome in patients, and describe a two-stage process that allows us to efficiently search for such patterns in large datasets. This involves first transforming continuous physiological signals from patients into symbolic sequences, and then searching for patterns in these reduced representations that are strongly associated with an outcome.Our strategy of identifying conserved activity that is unlikely to have occurred purely by chance in symbolic data is analogous to the discovery of regulatory motifs in genomic datasets. We build upon existing work in this area, generalizing the notion of a regulatory motif and enhancing current techniques to operate robustly on non-genomic data. We also address two significant considerations associated with motif discovery in general: computational efficiency and robustness in the presence of degeneracy and noise. To deal with these issues, we introduce the concept of active regions and new subset-based techniques such as a two-layer Gibbs sampling algorithm. These extensions allow for a framework for information inference, where precursors are identified as approximately conserved activity of arbitrary complexity preceding multiple occurrences of an event.We evaluated our solution on a population of patients who experienced sudden cardiac death and attempted to discover electrocardiographic activity that may be associated with the endpoint of death. To assess the predictive patterns discovered, we compared likelihood scores for motifs in the sudden death population against control populations of normal individuals and those with non-fatal supraventricular arrhythmias. Our results suggest that predictive motif discovery may be able to identify clinically relevant information even in the absence of significant prior knowledge.
Journal: Acm Transactions On Knowledge Discovery From Data, 2010 Jan DOI: 10.1145/1644873.1644875 PMID: 20730037.0 PMCID: PMC2923403
35. Sequences to systems.
Kellis, Rinn
A report of the seventh annual meeting on Systems Biology: Global Regulation of Gene Expression, 23-27 March 2010, Cold Spring Harbor, USA.
Journal: Genome Biology, 2010 DOI: 10.1186/gb-2010-11-5-303 PMID: 20500907.0 PMCID: PMC2898084
34. The consensus coding sequence (CCDS) project: Identifying a common protein-coding gene set for the human and mouse genomes.
Pruitt, Harrow, Harte, Wallin, Diekhans, Maglott, Searle, Farrell, Loveland, Ruef, Hart, Suner, Landrum, Aken, Ayling, Baertsch, Fernandez-Banet, Cherry, Curwen, Dicuccio, Kellis, Lee, Lin, Schuster, Shkeda, Amid, Brown, Dukhanina, Frankish, Hart, Maidak, Mudge, Murphy, Murphy, Rajan, Rajput, Riddick, Snow, Steward, Webb, Weber, Wilming, Wu, Birney, Haussler, Hubbard, Ostell, Durbin, Lipman
Effective use of the human and mouse genomes requires reliable identification of genes and their products. Although multiple public resources provide annotation, different methods are used that can result in similar but not identical representation of genes, transcripts, and proteins. The collaborative consensus coding sequence (CCDS) project tracks identical protein annotations on the reference mouse and human genomes with a stable identifier (CCDS ID), and ensures that they are consistently represented on the NCBI, Ensembl, and UCSC Genome Browsers. Importantly, the project coordinates on manually reviewing inconsistent protein annotations between sites, as well as annotations for which new evidence suggests a revision is needed, to progressively converge on a complete protein-coding set for the human and mouse reference genomes, while maintaining a high standard of reliability and biological accuracy. To date, the project has identified 20,159 human and 17,707 mouse consensus coding regions from 17,052 human and 16,893 mouse genes. Three evaluation methods indicate that the entries in the CCDS set are highly likely to represent real proteins, more so than annotations from contributing groups not included in CCDS. The CCDS database thus centralizes the function of identifying well-supported, identically-annotated, protein-coding regions.
Journal: Genome Research, 2009 Jul DOI: 10.1101/gr.080531.108 PMID: 19498102.0 PMCID: PMC2704439
33. Unlocking the secrets of the genome.
Celniker, Dillon, Gerstein, Gunsalus, Henikoff, Karpen, Kellis, Lai, Lieb, MacAlpine, Micklem, Piano, Snyder, Stein, White, Waterston
Despite the successes of genomics, little is known about how genetic information produces complex organisms. A look at the crucial functional elements of fly and worm genomes could change that. SUPPLEMENTARY INFORMATION: The online version of this article (doi:10.1038/459927a) contains supplementary material, which is available to authorized users.
Journal: Nature, 2009 Jun 18 DOI: 10.1038/459927a PMID: 19536255.0 PMCID: PMC2843545
32. Evolution of pathogenicity and sexual reproduction in eight Candida genomes.
Butler, Rasmussen, Lin, Santos, Sakthikumar, Munro, Rheinbay, Grabherr, Forche, Reedy, Agrafioti, Arnaud, Bates, Brown, Brunke, Costanzo, Fitzpatrick, de, Harris, Hoyer, Hube, Klis, Kodira, Lennard, Logue, Martin, Neiman, Nikolaou, Quail, Quinn, Santos, Schmitzberger, Sherlock, Shah, Silverstein, Skrzypek, Soll, Staggs, Stansfield, Stumpf, Sudbery, Srikantha, Zeng, Berman, Berriman, Heitman, Gow, Lorenz, Birren, Kellis, Cuomo
Candida species are the most common cause of opportunistic fungal infection worldwide. Here we report the genome sequences of six Candida species and compare these and related pathogens and non-pathogens. There are significant expansions of cell wall, secreted and transporter gene families in pathogenic species, suggesting adaptations associated with virulence. Large genomic tracts are homozygous in three diploid species, possibly resulting from recent recombination events. Surprisingly, key components of the mating and meiosis pathways are missing from several species. These include major differences at the mating-type loci (MTL); Lodderomyces elongisporus lacks MTL, and components of the a1/2 cell identity determinant were lost in other species, raising questions about how mating and cell types are controlled. Analysis of the CUG leucine-to-serine genetic-code change reveals that 99% of ancestral CUG codons were erased and new ones arose elsewhere. Lastly, we revise the Candida albicans gene catalogue, identifying many new genes.
Journal: Nature, 2009 Jun 4 DOI: 10.1038/nature08064 PMID: 19465905.0 PMCID: PMC2834264
31. Histone modifications at human enhancers reflect global cell-type-specific gene expression.
Heintzman, Hon, Hawkins, Kheradpour, Stark, Harp, Ye, Lee, Stuart, Ching, Ching, Antosiewicz-Bourget, Liu, Zhang, Green, Lobanenkov, Stewart, Thomson, Crawford, Kellis, Ren
The human body is composed of diverse cell types with distinct functions. Although it is known that lineage specification depends on cell-specific gene expression, which in turn is driven by promoters, enhancers, insulators and other cis-regulatory DNA sequences for each gene, the relative roles of these regulatory elements in this process are not clear. We have previously developed a chromatin-immunoprecipitation-based microarray method (ChIP-chip) to locate promoters, enhancers and insulators in the human genome. Here we use the same approach to identify these elements in multiple cell types and investigate their roles in cell-type-specific gene expression. We observed that the chromatin state at promoters and CTCF-binding at insulators is largely invariant across diverse cell types. In contrast, enhancers are marked with highly cell-type-specific histone modification patterns, strongly correlate to cell-type-specific gene expression programs on a global scale, and are functionally active in a cell-type-specific manner. Our results define over 55,000 potential transcriptional enhancers in the human genome, significantly expanding the current catalogue of human enhancers and highlighting the role of these elements in cell-type-specific gene expression.
Journal: Nature, 2009 May 7 DOI: 10.1038/nature07829 PMID: 19295514.0 PMCID: PMC2910248
30. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals.
Guttman, Amit, Garber, French, Lin, Feldser, Huarte, Zuk, Carey, Cassady, Cabili, Jaenisch, Mikkelsen, Jacks, Hacohen, Bernstein, Kellis, Regev, Rinn, Lander
There is growing recognition that mammalian cells produce many thousands of large intergenic transcripts. However, the functional significance of these transcripts has been particularly controversial. Although there are some well-characterized examples, most (>95%) show little evidence of evolutionary conservation and have been suggested to represent transcriptional noise. Here we report a new approach to identifying large non-coding RNAs using chromatin-state maps to discover discrete transcriptional units intervening known protein-coding loci. Our approach identified approximately 1,600 large multi-exonic RNAs across four mouse cell types. In sharp contrast to previous collections, these large intervening non-coding RNAs (lincRNAs) show strong purifying selection in their genomic loci, exonic sequences and promoter regions, with greater than 95% showing clear evolutionary conservation. We also developed a functional genomics approach that assigns putative functions to each lincRNA, demonstrating a diverse range of roles for lincRNAs in processes from embryonic stem cell pluripotency to cell proliferation. We obtained independent functional validation for the predictions for over 100 lincRNAs, using cell-based assays. In particular, we demonstrate that specific lincRNAs are transcriptionally regulated by key transcription factors in these processes such as p53, NFkappaB, Sox2, Oct4 (also known as Pou5f1) and Nanog. Together, these results define a unique collection of functional lincRNAs that are highly conserved and implicated in diverse biological processes.
Journal: Nature, 2009 Mar 12 DOI: 10.1038/nature07672 PMID: 19182780.0 PMCID: PMC2754849
29. An endogenous small interfering RNA pathway in Drosophila.
Czech, Malone, Zhou, Stark, Schlingeheyde, Dus, Perrimon, Kellis, Wohlschlegel, Sachidanandam, Hannon, Brennecke
Drosophila endogenous small RNAs are categorized according to their mechanisms of biogenesis and the Argonaute protein to which they bind. MicroRNAs are a class of ubiquitously expressed RNAs of approximately 22 nucleotides in length, which arise from structured precursors through the action of Drosha-Pasha and Dicer-1-Loquacious complexes. These join Argonaute-1 to regulate gene expression. A second endogenous small RNA class, the Piwi-interacting RNAs, bind Piwi proteins and suppress transposons. Piwi-interacting RNAs are restricted to the gonad, and at least a subset of these arises by Piwi-catalysed cleavage of single-stranded RNAs. Here we show that Drosophila generates a third small RNA class, endogenous small interfering RNAs, in both gonadal and somatic tissues. Production of these RNAs requires Dicer-2, but a subset depends preferentially on Loquacious rather than the canonical Dicer-2 partner, R2D2 (ref. 14). Endogenous small interfering RNAs arise both from convergent transcription units and from structured genomic loci in a tissue-specific fashion. They predominantly join Argonaute-2 and have the capacity, as a class, to target both protein-coding genes and mobile elements. These observations expand the repertoire of small RNAs in Drosophila, adding a class that blurs distinctions based on known biogenesis mechanisms and functional roles.
Journal: Nature, 2008 Jun 5 DOI: 10.1038/nature07007 PMID: 18463631.0 PMCID: PMC2895258
28. Conservation of small RNA pathways in platypus.
Murchison, Kheradpour, Sachidanandam, Smith, Hodges, Xuan, Kellis, Grutzner, Stark, Hannon
Small RNA pathways play evolutionarily conserved roles in gene regulation and defense from parasitic nucleic acids. The character and expression patterns of small RNAs show conservation throughout animal lineages, but specific animal clades also show variations on these recurring themes, including species-specific small RNAs. The monotremes, with only platypus and four species of echidna as extant members, represent the basal branch of the mammalian lineage. Here, we examine the small RNA pathways of monotremes by deep sequencing of six platypus and echidna tissues. We find that highly conserved microRNA species display their signature tissue-specific expression patterns. In addition, we find a large rapidly evolving cluster of microRNAs on platypus chromosome X1, which is unique to monotremes. Platypus and echidna testes contain a robust Piwi-interacting (piRNA) system, which appears to be participating in ongoing transposon defense.
Journal: Genome Research, 2008 Jun DOI: 10.1101/gr.073056.107 PMID: 18463306.0 PMCID: PMC2413167
27. Genome analysis of the platypus reveals unique signatures of evolution.
Warren, Hillier, Marshall, Birney, Ponting, Grutzner, Belov, Miller, Clarke, Chinwalla, Yang, Heger, Locke, Miethke, Waters, Veyrunes, Fulton, Fulton, Graves, Wallis, Puente, Lopez-Otin, Ordonez, Eichler, Chen, Cheng, Deakin, Alsop, Thompson, Kirby, Papenfuss, Wakefield, Olender, Lancet, Huttley, Smit, Pask, Temple-Smith, Batzer, Walker, Konkel, Harris, Whittington, Wong, Gemmell, Buschiazzo, Vargas, Merkel, Schmitz, Zemann, Churakov, Kriegs, Brosius, Murchison, Sachidanandam, Smith, Hannon, Tsend-Ayush, McMillan, Attenborough, Rens, Ferguson-Smith, Lefevre, Sharp, Nicholas, Ray, Kube, Reinhardt, Pringle, Taylor, Jones, Nixon, Dacheux, Niwa, Sekita, Huang, Stark, Kheradpour, Kellis, Flicek, Chen, Webber, Hardison, Nelson, Hallsworth-Pepin, Delehaunty, Markovic, Minx, Feng, Kremitzki, Mitreva, Glasscock, Wylie, Wohldmann, Thiru, Nhan, Pohl, Smith, Hou, Nefedov, de, Renfree, Mardis, Wilson
We present a draft genome sequence of the platypus, Ornithorhynchus anatinus. This monotreme exhibits a fascinating combination of reptilian and mammalian characters. For example, platypuses have a coat of fur adapted to an aquatic lifestyle; platypus females lactate, yet lay eggs; and males are equipped with venom similar to that of reptiles. Analysis of the first monotreme genome aligned these features with genetic innovations. We find that reptile and platypus venom proteins have been co-opted independently from the same gene families; milk protein genes are conserved despite platypuses laying eggs; and immune gene family expansions are directly related to platypus biology. Expansions of protein, non-protein-coding RNA and microRNA families, as well as repeat elements, are identified. Sequencing of this genome now provides a valuable resource for deep mammalian comparative analyses, as well as for monotreme biology and conservation.
Journal: Nature, 2008 May 8 DOI: 10.1038/nature06936 PMID: 18464734.0 PMCID: PMC2803040
26. Performance and scalability of discriminative metrics for comparative gene identification in 12 Drosophila genomes.
Lin, Deoras, Rasmussen, Kellis
Comparative genomics of multiple related species is a powerful methodology for the discovery of functional genomic elements, and its power should increase with the number of species compared. Here, we use 12 Drosophila genomes to study the power of comparative genomics metrics to distinguish between protein-coding and non-coding regions. First, we study the relative power of different comparative metrics and their relationship to single-species metrics. We find that even relatively simple multi-species metrics robustly outperform advanced single-species metrics, especially for shorter exons (< or =240 nt), which are common in animal genomes. Moreover, the two capture largely independent features of protein-coding genes, with different sensitivity/specificity trade-offs, such that their combinations lead to even greater discriminatory power. In addition, we study how discovery power scales with the number and phylogenetic distance of the genomes compared. We find that species at a broad range of distances are comparably effective informants for pairwise comparative gene identification, but that these are surpassed by multi-species comparisons at similar evolutionary divergence. In particular, while pairwise discovery power plateaued at larger distances and never outperformed the most advanced single-species metrics, multi-species comparisons continued to benefit even from the most distant species with no apparent saturation. Last, we find that genes in functional categories typically considered fast-evolving can nonetheless be recovered at very high rates using comparative methods. Our results have implications for comparative genomics analyses in any species, including the human.
Journal: Plos Computational Biology, 2008 Apr 18 DOI: 10.1371/journal.pcbi.1000067 PMID: 18421375.0 PMCID: PMC2291194
25. The evolutionary dynamics of the Saccharomyces cerevisiae protein interaction network after duplication.
Presser, Elowitz, Kellis, Kishony
Gene duplication is an important mechanism in the evolution of protein interaction networks. Duplications are followed by the gain and loss of interactions, rewiring the network at some unknown rate. Because rewiring is likely to change the distribution of network motifs within the duplicated interaction set, it should be possible to study network rewiring by tracking the evolution of these motifs. We have developed a mathematical framework that, together with duplication data from comparative genomic and proteomic studies, allows us to infer the connectivity of the preduplication network and the changes in connectivity over time. We focused on the whole-genome duplication (WGD) event in Saccharomyces cerevisiae. The model allowed us to predict the frequency of intergene interaction before WGD and the post duplication probabilities of interaction gain and loss. We find that the predicted frequency of self-interactions in the preduplication network is significantly higher than that observed in today's network. This could suggest a structural difference between the modern and ancestral networks, preferential addition or retention of interactions between ohnologs, or selective pressure to preserve duplicates of self-interacting proteins.
Journal: Proceedings Of The National Academy Of Sciences Of The United States Of America, 2008 Jan 22 DOI: 10.1073/pnas.0707293105 PMID: 18199840.0 PMCID: PMC2242688
24. A single Hox locus in Drosophila produces functional microRNAs from opposite DNA strands.
Stark, Bushati, Jan, Kheradpour, Hodges, Brennecke, Bartel, Cohen, Kellis
MicroRNAs (miRNAs) are approximately 22-nucleotide RNAs that are processed from characteristic precursor hairpins and pair to sites in messages of protein-coding genes to direct post-transcriptional repression. Here, we report that the miRNA iab-4 locus in the Drosophila Hox cluster is transcribed convergently from both DNA strands, giving rise to two distinct functional miRNAs. Both sense and antisense miRNA products target neighboring Hox genes via highly conserved sites, leading to homeotic transformations when ectopically expressed. We also report sense/antisense miRNAs in mouse and find antisense transcripts close to many miRNAs in both flies and mammals, suggesting that additional sense/antisense pairs exist.
Journal: Genes & Development, 2008 Jan 1 DOI: 10.1101/gad.1613108 PMID: 18172160.0 PMCID: PMC2151017
23. Distinguishing protein-coding and noncoding genes in the human genome.
Clamp, Fry, Kamal, Xie, Cuff, Lin, Kellis, Lindblad-Toh, Lander
Although the Human Genome Project was completed 4 years ago, the catalog of human protein-coding genes remains a matter of controversy. Current catalogs list a total of approximately 24,500 putative protein-coding genes. It is broadly suspected that a large fraction of these entries are functionally meaningless ORFs present by chance in RNA transcripts, because they show no evidence of evolutionary conservation with mouse or dog. However, there is currently no scientific justification for excluding ORFs simply because they fail to show evolutionary conservation: the alternative hypothesis is that most of these ORFs are actually valid human genes that reflect gene innovation in the primate lineage or gene loss in the other lineages. Here, we reject this hypothesis by carefully analyzing the nonconserved ORFs-specifically, their properties in other primates. We show that the vast majority of these ORFs are random occurrences. The analysis yields, as a by-product, a major revision of the current human catalogs, cutting the number of protein-coding genes to approximately 20,500. Specifically, it suggests that nonconserved ORFs should be added to the human gene catalog only if there is clear evidence of an encoded protein. It also provides a principled methodology for evaluating future proposed additions to the human gene catalog. Finally, the results indicate that there has been relatively little true innovation in mammalian protein-coding genes.
Journal: Proceedings Of The National Academy Of Sciences Of The United States Of America, 2007 Dec 4 DOI: 10.1073/pnas.0709013104 PMID: 18040051.0 PMCID: PMC2148306
22. Accurate gene-tree reconstruction by learning gene- and species-specific substitution rates across multiple complete genomes.
Rasmussen, Kellis
Comparative genomics provides a general methodology for discovering functional DNA elements and understanding their evolution. The availability of many related genomes enables more powerful analyses, but requires rigorous phylogenetic methods to resolve orthologous genes and regions. Here, we use 12 recently sequenced Drosophila genomes and nine fungal genomes to address the problem of accurate gene-tree reconstruction across many complete genomes. We show that existing phylogenetic methods that treat each gene tree in isolation show large-scale inaccuracies, largely due to insufficient phylogenetic information in individual genes. However, we find that gene trees exhibit common properties that can be exploited for evolutionary studies and accurate phylogenetic reconstruction. Evolutionary rates can be decoupled into gene-specific and species-specific components, which can be learned across complete genomes. We develop a phylogenetic reconstruction methodology that exploits these properties and achieves significantly higher accuracy, addressing the species-level heterotachy and enabling studies of gene evolution in the context of species evolution.
Journal: Genome Research, 2007 Dec DOI: 10.1101/gr.7105007 PMID: 17989260.0 PMCID: PMC2099600
21. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo.
Zeitlinger, Stark, Kellis, Hong, Nechaev, Adelman, Levine, Young
It is widely assumed that the key rate-limiting step in gene activation is the recruitment of RNA polymerase II (Pol II) to the core promoter. Although there are well-documented examples in which Pol II is recruited to a gene but stalls, a general role for Pol II stalling in development has not been established. We have carried out comprehensive Pol II chromatin immunoprecipitation microarray (ChIP-chip) assays in Drosophila embryos and identified three distinct Pol II binding behaviors: active (uniform binding across the entire transcription unit), no binding, and stalled (binding at the transcription start site). The notable feature of the approximately 10% genes that are stalled is that they are highly enriched for developmental control genes, which are either repressed or poised for activation during later stages of embryogenesis. We propose that Pol II stalling facilitates rapid temporal and spatial changes in gene activity during development.
Journal: Nature Genetics, 2007 Dec DOI: 10.1038/ng.2007.26 PMID: 17994019.0 PMCID: PMC2824921
20. Evolution, biogenesis, expression, and target predictions of a substantially expanded set of Drosophila microRNAs.
Ruby, Stark, Johnston, Kellis, Bartel, Lai
MicroRNA (miRNA) genes give rise to small regulatory RNAs in a wide variety of organisms. We used computational methods to predict miRNAs conserved among Drosophila species and large-scale sequencing of small RNAs from Drosophila melanogaster to experimentally confirm and complement these predictions. In addition to validating 20 of our top 45 predictions for novel miRNA loci, the large-scale sequencing identified many miRNAs that had not been predicted. In total, 59 novel genes were identified, increasing our tally of confirmed fly miRNAs to 148. The large-scale sequencing also refined the identities of previously known miRNAs and provided insights into their biogenesis and expression. Many miRNAs were expressed in particular developmental contexts, with a large cohort of miRNAs expressed primarily in imaginal discs. Conserved miRNAs typically were expressed more broadly and robustly than were nonconserved miRNAs, and those conserved miRNAs with more restricted expression tended to have fewer predicted targets than those expressed more broadly. Predicted targets for the expanded set of microRNAs substantially increased and revised the miRNA-target relationships that appear conserved among the fly species. Insights were also provided into miRNA gene evolution, including evidence for emergent regulatory function deriving from the opposite arm of the miRNA hairpin, exemplified by mir-10, and even the opposite strand of the DNA, exemplified by mir-iab-4.
Journal: Genome Research, 2007 Dec DOI: 10.1101/gr.6597907 PMID: 17989254.0 PMCID: PMC2099593
19. Revisiting the protein-coding gene catalog of Drosophila melanogaster using 12 fly genomes.
Lin, Carlson, Crosby, Matthews, Yu, Park, Wan, Schroeder, Gramates, St, Roark, Wiley, Kulathinal, Zhang, Myrick, Antone, Celniker, Gelbart, Kellis
The availability of sequenced genomes from 12 Drosophila species has enabled the use of comparative genomics for the systematic discovery of functional elements conserved within this genus. We have developed quantitative metrics for the evolutionary signatures specific to protein-coding regions and applied them genome-wide, resulting in 1193 candidate new protein-coding exons in the D. melanogaster genome. We have reviewed these predictions by manual curation and validated a subset by directed cDNA screening and sequencing, revealing both new genes and new alternative splice forms of known genes. We also used these evolutionary signatures to evaluate existing gene annotations, resulting in the validation of 87% of genes lacking descriptive names and identifying 414 poorly conserved genes that are likely to be spurious predictions, noncoding, or species-specific genes. Furthermore, our methods suggest a variety of refinements to hundreds of existing gene models, such as modifications to translation start codons and exon splice boundaries. Finally, we performed directed genome-wide searches for unusual protein-coding structures, discovering 149 possible examples of stop codon readthrough, 125 new candidate ORFs of polycistronic mRNAs, and several candidate translational frameshifts. These results affect >10% of annotated fly genes and demonstrate the power of comparative genomics to enhance our understanding of genome organization, even in a model organism as intensively studied as Drosophila melanogaster.
Journal: Genome Research, 2007 Dec DOI: 10.1101/gr.6679507 PMID: 17989253.0 PMCID: PMC2099591
18. Reliable prediction of regulator targets using 12 Drosophila genomes.
Kheradpour, Stark, Roy, Kellis
Gene expression is regulated pre- and post-transcriptionally via cis-regulatory DNA and RNA motifs. Identification of individual functional instances of such motifs in genome sequences is a major goal for inferring regulatory networks yet has been hampered due to the motifs' short lengths that lead to many chance matches and poor signal-to-noise ratios. In this paper, we develop a general methodology for the comparative identification of functional motif instances across many related species, using a phylogenetic framework that accounts for the evolutionary relationships between species, allows for motif movements, and is robust against missing data due to artifacts in sequencing, assembly, or alignment. We also provide a robust statistical framework for evaluating motif confidence, which enables us to translate evolutionary conservation into a confidence measure for each motif instance, correcting for varying motif length, composition, and background conservation of the target regions. We predict targets of fly transcription factors and miRNAs in alignments of 12 recently sequenced Drosophila species. When compared to extensive genome-wide experimental data, predicted targets are of high quality, matching and surpassing ChIP-chip microarrays and recovering miRNA targets with high sensitivity. The resulting regulatory network suggests significant redundancy between pre- and post-transcriptional regulation of gene expression.
Journal: Genome Research, 2007 Dec DOI: 10.1101/gr.7090407 PMID: 17989251.0 PMCID: PMC2099599
17. Systematic discovery and characterization of fly microRNAs using 12 Drosophila genomes.
Stark, Kheradpour, Parts, Brennecke, Hodges, Hannon, Kellis
MicroRNAs (miRNAs) are short regulatory RNAs that inhibit target genes by complementary binding in 3' untranslated regions (3' UTRs). They are one of the most abundant classes of regulators, targeting a large fraction of all genes, making their comprehensive study a requirement for understanding regulation and development. Here we use 12 Drosophila genomes to define structural and evolutionary signatures of miRNA hairpins, which we use for their de novo discovery. We predict >41 novel miRNA genes, which encompass many unique families, and 28 of which are validated experimentally. We also define signals for the precise start position of mature miRNAs, which suggest corrections of previously known miRNAs, often leading to drastic changes in their predicted target spectrum. We show that miRNA discovery power scales with the number and divergence of species compared, suggesting that such approaches can be successful in human as dozens of mammalian genomes become available. Interestingly, for some miRNAs sense and anti-sense hairpins score highly and mature miRNAs from both strands can indeed be found in vivo. Similarly, miRNAs with weak 5' end predictions show increased in vivo processing of multiple alternate 5' ends and have fewer predicted targets. Lastly, we show that several miRNA star sequences score highly and are likely functional. For mir-10 in particular, both arms show abundant processing, and both show highly conserved target sites in Hox genes, suggesting a possible cooperation of the two arms, and their role as a master Hox regulator.
Journal: Genome Research, 2007 Dec DOI: 10.1101/gr.6593807 PMID: 17989255.0 PMCID: PMC2099594
16. Evolution of genes and genomes on the Drosophila phylogeny.
Clark, Eisen, Smith, Bergman, Oliver, Markow, Kaufman, Kellis, Gelbart, Iyer, Pollard, Sackton, Larracuente, Singh, Abad, Abt, Adryan, Aguade, Akashi, Anderson, Aquadro, Ardell, Arguello, Artieri, Barbash, Barker, Barsanti, Batterham, Batzoglou, Begun, Bhutkar, Blanco, Bosak, Bradley, Brand, Brent, Brooks, Brown, Butlin, Caggese, Calvi, Bernardo, Caspi, Castrezana, Celniker, Chang, Chapple, Chatterji, Chinwalla, Civetta, Clifton, Comeron, Costello, Coyne, Daub, David, Delcher, Delehaunty, Do, Ebling, Edwards, Eickbush, Evans, Filipski, Findeiss, Freyhult, Fulton, Fulton, Garcia, Gardiner, Garfield, Garvin, Gibson, Gilbert, Gnerre, Godfrey, Good, Gotea, Gravely, Greenberg, Griffiths-Jones, Gross, Guigo, Gustafson, Haerty, Hahn, Halligan, Halpern, Halter, Han, Heger, Hillier, Hinrichs, Holmes, Hoskins, Hubisz, Hultmark, Huntley, Jaffe, Jagadeeshan, Jeck, Johnson, Jones, Jordan, Karpen, Kataoka, Keightley, Kheradpour, Kirkness, Koerich, Kristiansen, Kudrna, Kulathinal, Kumar, Kwok, Lander, Langley, Lapoint, Lazzaro, Lee, Levesque, Li, Lin, Lin, Lindblad-Toh, Llopart, Long, Low, Lozovsky, Lu, Luo, Machado, Makalowski, Marzo, Matsuda, Matzkin, McAllister, McBride, McKernan, McKernan, Mendez-Lago, Minx, Mollenhauer, Montooth, Mount, Mu, Myers, Negre, Newfeld, Nielsen, Noor, O'Grady, Pachter, Papaceit, Parisi, Parisi, Parts, Pedersen, Pesole, Phillippy, Ponting, Pop, Porcelli, Powell, Prohaska, Pruitt, Puig, Quesneville, Ram, Rand, Rasmussen, Reed, Reenan, Reily, Remington, Rieger, Ritchie, Robin, Rogers, Rohde, Rozas, Rubenfield, Ruiz, Russo, Salzberg, Sanchez-Gracia, Saranga, Sato, Schaeffer, Schatz, Schlenke, Schwartz, Segarra, Singh, Sirot, Sirota, Sisneros, Smith, Smith, Spieth, Stage, Stark, Stephan, Strausberg, Strempel, Sturgill, Sutton, Sutton, Tao, Teichmann, Tobari, Tomimura, Tsolas, Valente, Venter, Venter, Vicario, Vieira, Vilella, Villasante, Walenz, Wang, Wasserman, Watts, Wilson, Wilson, Wing, Wolfner, Wong, Wong, Wu, Wu, Yamamoto, Yang, Yang, Yorke, Yoshida, Zdobnov, Zhang, Zhang, Zimin, Baldwin, Abdouelleil, Abdulkadir, Abebe, Abera, Abreu, Acer, Aftuck, Alexander, An, Anderson, Anderson, Arachi, Azer, Bachantsang, Barry, Bayul, Berlin, Bessette, Bloom, Blye, Boguslavskiy, Bonnet, Boukhgalter, Bourzgui, Brown, Cahill, Channer, Cheshatsang, Chuda, Citroen, Collymore, Cooke, Costello, D'Aco, Daza, De, DeGray, DeMaso, Dhargay, Dooley, Dooley, Doricent, Dorje, Dorjee, Dupes, Elong, Falk, Farina, Faro, Ferguson, Fisher, Foley, Franke, Friedrich, Gadbois, Gearin, Gearin, Giannoukos, Goode, Graham, Grandbois, Grewal, Gyaltsen, Hafez, Hagos, Hall, Henson, Hollinger, Honan, Huard, Hughes, Hurhula, Husby, Kamat, Kanga, Kashin, Khazanovich, Kisner, Lance, Lara, Lee, Lennon, Letendre, LeVine, Lipovsky, Liu, Liu, Liu, Lokyitsang, Lokyitsang, Lubonja, Lui, MacDonald, Magnisalis, Maru, Matthews, McCusker, McDonough, Mehta, Meldrim, Meneus, Mihai, Mihalev, Mihova, Mittelman, Mlenga, Montmayeur, Mulrain, Navidi, Naylor, Negash, Nguyen, Nguyen, Nicol, Norbu, Norbu, Novod, O'Neill, Osman, Markiewicz, Oyono, Patti, Phunkhang, Pierre, Priest, Raghuraman, Rege, Reyes, Rise, Rogov, Ross, Ryan, Settipalli, Shea, Sherpa, Shi, Shih, Sparrow, Spaulding, Stalker, Stange-Thomann, Stavropoulos, Stone, Strader, Tesfaye, Thomson, Thoulutsang, Thoulutsang, Topham, Topping, Tsamla, Vassiliev, Vo, Wangchuk, Wangdi, Weiand, Wilkinson, Wilson, Yadav, Young, Yu, Zembek, Zhong, Zimmer, Zwirko, Jaffe, Alvarez, Brockman, Butler, Chin, Gnerre, Grabherr, Kleber, Mauceli, MacCallum
Comparative analysis of multiple genomes in a phylogenetic framework dramatically improves the precision and sensitivity of evolutionary inference, producing more robust results than single-genome analyses can provide. The genomes of 12 Drosophila species, ten of which are presented here for the first time (sechellia, simulans, yakuba, erecta, ananassae, persimilis, willistoni, mojavensis, virilis and grimshawi), illustrate how rates and patterns of sequence divergence across taxa can illuminate evolutionary processes on a genomic scale. These genome sequences augment the formidable genetic tools that have made Drosophila melanogaster a pre-eminent model for animal genetics, and will further catalyse fundamental research on mechanisms of development, cell biology, genetics, disease, neurobiology, behaviour, physiology and evolution. Despite remarkable similarities among these Drosophila species, we identified many putatively non-neutral changes in protein-coding genes, non-coding RNA genes, and cis-regulatory regions. These may prove to underlie differences in the ecology and behaviour of these diverse species.
Journal: Nature, 2007 Nov 8 DOI: 10.1038/nature06341 PMID: 17994087.0
15. Discovery of functional elements in 12 Drosophila genomes using evolutionary signatures.
Stark, Lin, Kheradpour, Pedersen, Parts, Carlson, Crosby, Rasmussen, Roy, Deoras, Ruby, Brennecke, Hodges, Hinrichs, Caspi, Paten, Park, Han, Maeder, Polansky, Robson, Aerts, van, Hassan, Gilbert, Eastman, Rice, Weir, Hahn, Park, Dewey, Pachter, Kent, Haussler, Lai, Bartel, Hannon, Kaufman, Eisen, Clark, Smith, Celniker, Gelbart, Kellis
Sequencing of multiple related species followed by comparative genomics analysis constitutes a powerful approach for the systematic understanding of any genome. Here, we use the genomes of 12 Drosophila species for the de novo discovery of functional elements in the fly. Each type of functional element shows characteristic patterns of change, or 'evolutionary signatures', dictated by its precise selective constraints. Such signatures enable recognition of new protein-coding genes and exons, spurious and incorrect gene annotations, and numerous unusual gene structures, including abundant stop-codon readthrough. Similarly, we predict non-protein-coding RNA genes and structures, and new microRNA (miRNA) genes. We provide evidence of miRNA processing and functionality from both hairpin arms and both DNA strands. We identify several classes of pre- and post-transcriptional regulatory motifs, and predict individual motif instances with high confidence. We also study how discovery power scales with the divergence and number of species compared, and we provide general guidelines for comparative studies.
Journal: Nature, 2007 Nov 8 DOI: 10.1038/nature06340 PMID: 17994088.0 PMCID: PMC2474711
14. Systematic discovery of regulatory motifs in conserved regions of the human genome, including thousands of CTCF insulator sites.
Xie, Mikkelsen, Gnirke, Lindblad-Toh, Kellis, Lander
Conserved noncoding elements (CNEs) constitute the majority of sequences under purifying selection in the human genome, yet their function remains largely unknown. Experimental evidence suggests that many of these elements play regulatory roles, but little is known about regulatory motifs contained within them. Here we describe a systematic approach to discover and characterize regulatory motifs within mammalian CNEs by searching for long motifs (12-22 nt) with significant enrichment in CNEs and studying their biochemical and genomic properties. Our analysis identifies 233 long motifs (LMs), matching a total of approximately 60,000 conserved instances across the human genome. These motifs include 16 previously known regulatory elements, such as the histone 3'-UTR motif and the neuron-restrictive silencer element, as well as striking examples of novel functional elements. The most highly enriched motif (LM1) corresponds to the X-box motif known from yeast and nematode. We show that it is bound by the RFX1 protein and identify thousands of conserved motif instances, suggesting a broad role for the RFX family in gene regulation. A second group of motifs (LM2*) does not match any previously known motif. We demonstrate by biochemical and computational methods that it defines a binding site for the CTCF protein, which is involved in insulator function to limit the spread of gene activation. We identify nearly 15,000 conserved sites that likely serve as insulators, and we show that nearby genes separated by predicted CTCF sites show markedly reduced correlation in gene expression. These sites may thus partition the human genome into domains of expression.
Journal: Proceedings Of The National Academy Of Sciences Of The United States Of America, 2007 Apr 24 DOI: 10.1073/pnas.0701811104 PMID: 17442748.0 PMCID: PMC1852749
13. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila.
Brennecke, Aravin, Stark, Dus, Kellis, Sachidanandam, Hannon
Drosophila Piwi-family proteins have been implicated in transposon control. Here, we examine piwi-interacting RNAs (piRNAs) associated with each Drosophila Piwi protein and find that Piwi and Aubergine bind RNAs that are predominantly antisense to transposons, whereas Ago3 complexes contain predominantly sense piRNAs. As in mammals, the majority of Drosophila piRNAs are derived from discrete genomic loci. These loci comprise mainly defective transposon sequences, and some have previously been identified as master regulators of transposon activity. Our data suggest that heterochromatic piRNA loci interact with potentially active, euchromatic transposons to form an adaptive system for transposon control. Complementary relationships between sense and antisense piRNA populations suggest an amplification loop wherein each piRNA-directed cleavage event generates the 5' end of a new piRNA. Thus, sense piRNAs, formed following cleavage of transposon mRNAs may enhance production of antisense piRNAs, complementary to active elements, by directing cleavage of transcripts from master control loci.
Journal: Cell, 2007 Mar 23 DOI: 10.1016/j.cell.2007.01.043 PMID: 17346786.0
12. Whole-genome ChIP-chip analysis of Dorsal, Twist, and Snail suggests integration of diverse patterning processes in the Drosophila embryo.
Zeitlinger, Zinzen, Stark, Kellis, Zhang, Young, Levine
Genetic studies have identified numerous sequence-specific transcription factors that control development, yet little is known about their in vivo distribution across animal genomes. We determined the genome-wide occupancy of the dorsoventral (DV) determinants Dorsal, Twist, and Snail in the Drosophila embryo using chromatin immunoprecipitation coupled with microarray analysis (ChIP-chip). The in vivo binding of these proteins correlate tightly with the limits of known enhancers. Our analysis predicts substantially more target genes than previous estimates, and includes Dpp signaling components and anteroposterior (AP) segmentation determinants. Thus, the ChIP-chip data uncover a much larger than expected regulatory network, which integrates diverse patterning processes during development.
Journal: Genes & Development, 2007 Feb 15 DOI: 10.1101/gad.1509607 PMID: 17322397.0 PMCID: PMC1804326
11. Genome sequence, comparative analysis and haplotype structure of the domestic dog.
Lindblad-Toh, Wade, Mikkelsen, Karlsson, Jaffe, Kamal, Clamp, Chang, Kulbokas, Zody, Mauceli, Xie, Breen, Wayne, Ostrander, Ponting, Galibert, Smith, DeJong, Kirkness, Alvarez, Biagi, Brockman, Butler, Chin, Cook, Cuff, Daly, DeCaprio, Gnerre, Grabherr, Kellis, Kleber, Bardeleben, Goodstadt, Heger, Hitte, Kim, Koepfli, Parker, Pollinger, Searle, Sutter, Thomas, Webber, Baldwin, Abebe, Abouelleil, Aftuck, Ait-Zahra, Aldredge, Allen, An, Anderson, Antoine, Arachchi, Aslam, Ayotte, Bachantsang, Barry, Bayul, Benamara, Berlin, Bessette, Blitshteyn, Bloom, Blye, Boguslavskiy, Bonnet, Boukhgalter, Brown, Cahill, Calixte, Camarata, Cheshatsang, Chu, Citroen, Collymore, Cooke, Dawoe, Daza, Decktor, DeGray, Dhargay, Dooley, Dooley, Dorje, Dorjee, Dorris, Duffey, Dupes, Egbiremolen, Elong, Falk, Farina, Faro, Ferguson, Ferreira, Fisher, FitzGerald, Foley, Foley, Franke, Friedrich, Gage, Garber, Gearin, Giannoukos, Goode, Goyette, Graham, Grandbois, Gyaltsen, Hafez, Hagopian, Hagos, Hall, Healy, Hegarty, Honan, Horn, Houde, Hughes, Hunnicutt, Husby, Jester, Jones, Kamat, Kanga, Kells, Khazanovich, Kieu, Kisner, Kumar, Lance, Landers, Lara, Lee, Leger, Lennon, Leuper, LeVine, Liu, Liu, Lokyitsang, Lokyitsang, Lui, Macdonald, Major, Marabella, Maru, Matthews, McDonough, Mehta, Meldrim, Melnikov, Meneus, Mihalev, Mihova, Miller, Mittelman, Mlenga, Mulrain, Munson, Navidi, Naylor, Nguyen, Nguyen, Nguyen, Nguyen, Nicol, Norbu, Norbu, Novod, Nyima, Olandt, O'Neill, O'Neill, Osman, Oyono, Patti, Perrin, Phunkhang, Pierre, Priest, Rachupka, Raghuraman, Rameau, Ray, Raymond, Rege, Rise, Rogers, Rogov, Sahalie, Settipalli, Sharpe, Shea, Sheehan, Sherpa, Shi, Shih, Sloan, Smith, Sparrow, Stalker, Stange-Thomann, Stavropoulos, Stone, Stone, Sykes, Tchuinga, Tenzing, Tesfaye, Thoulutsang, Thoulutsang, Topham, Topping, Tsamla, Vassiliev, Venkataraman, Vo, Wangchuk, Wangdi, Weiand, Wilkinson, Wilson, Yadav, Yang, Yang, Young, Yu, Zainoun, Zembek, Zimmer, Lander
Here we report a high-quality draft genome sequence of the domestic dog (Canis familiaris), together with a dense map of single nucleotide polymorphisms (SNPs) across breeds. The dog is of particular interest because it provides important evolutionary information and because existing breeds show great phenotypic diversity for morphological, physiological and behavioural traits. We use sequence comparison with the primate and rodent lineages to shed light on the structure and evolution of genomes and genes. Notably, the majority of the most highly conserved non-coding sequences in mammalian genomes are clustered near a small subset of genes with important roles in development. Analysis of SNPs reveals long-range haplotypes across the entire dog genome, and defines the nature of genetic diversity within and across breeds. The current SNP map now makes it possible for genome-wide association studies to identify genes responsible for diseases and traits, with important consequences for human and companion animal health.
Journal: Nature, 2005 Dec 8 DOI: 10.1038/nature04338 PMID: 16341006.0
10. Systematic discovery of regulatory motifs in human promoters and 3' UTRs by comparison of several mammals.
Xie, Lu, Kulbokas, Golub, Mootha, Lindblad-Toh, Lander, Kellis
Comprehensive identification of all functional elements encoded in the human genome is a fundamental need in biomedical research. Here, we present a comparative analysis of the human, mouse, rat and dog genomes to create a systematic catalogue of common regulatory motifs in promoters and 3' untranslated regions (3' UTRs). The promoter analysis yields 174 candidate motifs, including most previously known transcription-factor binding sites and 105 new motifs. The 3'-UTR analysis yields 106 motifs likely to be involved in post-transcriptional regulation. Nearly one-half are associated with microRNAs (miRNAs), leading to the discovery of many new miRNA genes and their likely target genes. Our results suggest that previous estimates of the number of human miRNA genes were low, and that miRNAs regulate at least 20% of human genes. The overall results provide a systematic view of gene regulation in the human, which will be refined as additional mammalian genomes become available.
Journal: Nature, 2005 Mar 17 DOI: 10.1038/nature03441 PMID: 15735639.0 PMCID: PMC2923337
9. Large-scale discovery and validation of functional elements in the human genome.
Bernstein, Kellis
A report on the genomics workshop 'Identification of Functional Elements in Mammalian Genomes', Cold Spring Harbor, New York, 11-13 November 2004.
Journal: Genome Biology, 2005 DOI: 10.1186/gb-2005-6-3-312 PMID: 15774039.0 PMCID: PMC1088940
8. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype.
Jaillon, Aury, Brunet, Petit, Stange-Thomann, Mauceli, Bouneau, Fischer, Ozouf-Costaz, Bernot, Nicaud, Jaffe, Fisher, Lutfalla, Dossat, Segurens, Dasilva, Salanoubat, Levy, Boudet, Castellano, Anthouard, Jubin, Castelli, Katinka, Vacherie, Biemont, Skalli, Cattolico, Poulain, De, Cruaud, Duprat, Brottier, Coutanceau, Gouzy, Parra, Lardier, Chapple, McKernan, McEwan, Bosak, Kellis, Volff, Guigo, Zody, Mesirov, Lindblad-Toh, Birren, Nusbaum, Kahn, Robinson-Rechavi, Laudet, Schachter, Quetier, Saurin, Scarpelli, Wincker, Lander, Weissenbach, Roest
Tetraodon nigroviridis is a freshwater puffer fish with the smallest known vertebrate genome. Here, we report a draft genome sequence with long-range linkage and substantial anchoring to the 21 Tetraodon chromosomes. Genome analysis provides a greatly improved fish gene catalogue, including identifying key genes previously thought to be absent in fish. Comparison with other vertebrates and a urochordate indicates that fish proteins have diverged markedly faster than their mammalian homologues. Comparison with the human genome suggests approximately 900 previously unannotated human genes. Analysis of the Tetraodon and human genomes shows that whole-genome duplication occurred in the teleost fish lineage, subsequent to its divergence from mammals. The analysis also makes it possible to infer the basic structure of the ancestral bony vertebrate genome, which was composed of 12 chromosomes, and to reconstruct much of the evolutionary history of ancient and recent chromosome rearrangements leading to the modern human karyotype.
Journal: Nature, 2004 Oct 21 DOI: 10.1038/nature03025 PMID: 15496914.0
7. Transcriptional regulatory code of a eukaryotic genome.
Harbison, Gordon, Lee, Rinaldi, Macisaac, Danford, Hannett, Tagne, Reynolds, Yoo, Jennings, Zeitlinger, Pokholok, Kellis, Rolfe, Takusagawa, Lander, Gifford, Fraenkel, Young
DNA-binding transcriptional regulators interpret the genome's regulatory code by binding to specific sequences to induce or repress gene expression. Comparative genomics has recently been used to identify potential cis-regulatory sequences within the yeast genome on the basis of phylogenetic conservation, but this information alone does not reveal if or when transcriptional regulators occupy these binding sites. We have constructed an initial map of yeast's transcriptional regulatory code by identifying the sequence elements that are bound by regulators under various conditions and that are conserved among Saccharomyces species. The organization of regulatory elements in promoters and the environment-dependent use of these elements by regulators are discussed. We find that environment-specific use of regulatory elements predicts mechanistic models for the function of a large population of yeast's transcriptional regulators.
Journal: Nature, 2004 Sep 2 DOI: 10.1038/nature02800 PMID: 15343339.0 PMCID: PMC3006441
6. Proof and evolutionary analysis of ancient genome duplication in the yeast Saccharomyces cerevisiae.
Kellis, Birren, Lander
Whole-genome duplication followed by massive gene loss and specialization has long been postulated as a powerful mechanism of evolutionary innovation. Recently, it has become possible to test this notion by searching complete genome sequence for signs of ancient duplication. Here, we show that the yeast Saccharomyces cerevisiae arose from ancient whole-genome duplication, by sequencing and analysing Kluyveromyces waltii, a related yeast species that diverged before the duplication. The two genomes are related by a 1:2 mapping, with each region of K. waltii corresponding to two regions of S. cerevisiae, as expected for whole-genome duplication. This resolves the long-standing controversy on the ancestry of the yeast genome, and makes it possible to study the fate of duplicated genes directly. Strikingly, 95% of cases of accelerated evolution involve only one member of a gene pair, providing strong support for a specific model of evolution, and allowing us to distinguish ancestral and derived functions.
Journal: Nature, 2004 Apr 8 DOI: 10.1038/nature02424 PMID: 15004568.0
5. Methods in comparative genomics: genome correspondence, gene identification and regulatory motif discovery.
Kellis, Patterson, Birren, Berger, Lander
In Kellis et al. (2003), we reported the genome sequences of S. paradoxus, S. mikatae, and S. bayanus and compared these three yeast species to their close relative, S. cerevisiae. Genomewide comparative analysis allowed the identification of functionally important sequences, both coding and noncoding. In this companion paper we describe the mathematical and algorithmic results underpinning the analysis of these genomes. (1) We present methods for the automatic determination of genome correspondence. The algorithms enabled the automatic identification of orthologs for more than 90% of genes and intergenic regions across the four species despite the large number of duplicated genes in the yeast genome. The remaining ambiguities in the gene correspondence revealed recent gene family expansions in regions of rapid genomic change. (2) We present methods for the identification of protein-coding genes based on their patterns of nucleotide conservation across related species. We observed the pressure to conserve the reading frame of functional proteins and developed a test for gene identification with high sensitivity and specificity. We used this test to revisit the genome of S. cerevisiae, reducing the overall gene count by 500 genes (10% of previously annotated genes) and refining the gene structure of hundreds of genes. (3) We present novel methods for the systematic de novo identification of regulatory motifs. The methods do not rely on previous knowledge of gene function and in that way differ from the current literature on computational motif discovery. Based on genomewide conservation patterns of known motifs, we developed three conservation criteria that we used to discover novel motifs. We used an enumeration approach to select strongly conserved motif cores, which we extended and collapsed into a small number of candidate regulatory motifs. These include most previously known regulatory motifs as well as several noteworthy novel motifs. The majority of discovered motifs are enriched in functionally related genes, allowing us to infer a candidate function for novel motifs. Our results demonstrate the power of comparative genomics to further our understanding of any species. Our methods are validated by the extensive experimental knowledge in yeast and will be invaluable in the study of complex genomes like that of the human.
Journal: Journal Of Computational Biology, 2004 DOI: 10.1089/1066527041410319 PMID: 15285895.0
4. The changing face of genomics.
Kellis
A report on the 5th annual Advances in Genome Biology and Technology (AGBT) and Automation in DNA Mapping and Sequencing (AMS) meeting, Marco Island, USA, 4-7 February 2004.
Journal: Genome Biology, 2004 DOI: 10.1186/gb-2004-5-5-324 PMID: 15128443.0 PMCID: PMC416465
3. Position specific variation in the rate of evolution in transcription factor binding sites.
Moses, Chiang, Kellis, Lander, Eisen
The binding sites of sequence specific transcription factors are an important and relatively well-understood class of functional non-coding DNAs. Although a wide variety of experimental and computational methods have been developed to characterize transcription factor binding sites, they remain difficult to identify. Comparison of non-coding DNA from related species has shown considerable promise in identifying these functional non-coding sequences, even though relatively little is known about their evolution. RESULTS: Here we analyse the genome sequences of the budding yeasts Saccharomyces cerevisiae, S. bayanus, S. paradoxus and S. mikatae to study the evolution of transcription factor binding sites. As expected, we find that both experimentally characterized and computationally predicted binding sites evolve slower than surrounding sequence, consistent with the hypothesis that they are under purifying selection. We also observe position-specific variation in the rate of evolution within binding sites. We find that the position-specific rate of evolution is positively correlated with degeneracy among binding sites within S. cerevisiae. We test theoretical predictions for the rate of evolution at positions where the base frequencies deviate from background due to purifying selection and find reasonable agreement with the observed rates of evolution. Finally, we show how the evolutionary characteristics of real binding motifs can be used to distinguish them from artefacts of computational motif finding algorithms. CONCLUSION: As has been observed for protein sequences, the rate of evolution in transcription factor binding sites varies with position, suggesting that some regions are under stronger functional constraint than others. This variation likely reflects the varying importance of different positions in the formation of the protein-DNA complex. The characterization of the pattern of evolution in known binding sites will likely contribute to the effective use of comparative sequence data in the identification of transcription factor binding sites and is an important step toward understanding the evolution of functional non-coding DNA.
Journal: Bmc Evolutionary Biology, 2003 Aug 28 DOI: 10.1186/1471-2148-3-19 PMID: 12946282.0 PMCID: PMC212491
2. Sequencing and comparison of yeast species to identify genes and regulatory elements.
Kellis, Patterson, Endrizzi, Birren, Lander
Identifying the functional elements encoded in a genome is one of the principal challenges in modern biology. Comparative genomics should offer a powerful, general approach. Here, we present a comparative analysis of the yeast Saccharomyces cerevisiae based on high-quality draft sequences of three related species (S. paradoxus, S. mikatae and S. bayanus). We first aligned the genomes and characterized their evolution, defining the regions and mechanisms of change. We then developed methods for direct identification of genes and regulatory motifs. The gene analysis yielded a major revision to the yeast gene catalogue, affecting approximately 15% of all genes and reducing the total count by about 500 genes. The motif analysis automatically identified 72 genome-wide elements, including most known regulatory motifs and numerous new motifs. We inferred a putative function for most of these motifs, and provided insights into their combinatorial interactions. The results have implications for genome analysis of diverse organisms, including the human.
Journal: Nature, 2003 May 15 DOI: 10.1038/nature01644 PMID: 12748633.0
1. Phylogenetically and spatially conserved word pairs associated with gene-expression changes in yeasts.
Chiang, Moses, Kellis, Lander, Eisen
Transcriptional regulation in eukaryotes often involves multiple transcription factors binding to the same transcription control region, and to understand the regulatory content of eukaryotic genomes it is necessary to consider the co-occurrence and spatial relationships of individual binding sites. The determination of conserved sequences (often known as phylogenetic footprinting) has identified individual transcription factor binding sites. We extend this concept of functional conservation to higher-order features of transcription control regions. RESULTS: We used the genome sequences of four yeast species of the genus Saccharomyces to identify sequences potentially involved in multifactorial control of gene expression. We found 989 potential regulatory 'templates': pairs of hexameric sequences that are jointly conserved in transcription regulatory regions and also exhibit non-random relative spacing. Many of the individual sequences in these templates correspond to known transcription factor binding sites, and the sets of genes containing a particular template in their transcription control regions tend to be differentially expressed in conditions where the corresponding transcription factors are known to be active. The incorporation of word pairs to define sequence features yields more specific predictions of average expression profiles and more informative regression models for genome-wide expression data than considering sequence conservation alone. CONCLUSIONS: The incorporation of both joint conservation and spacing constraints of sequence pairs predicts groups of target genes that are specific for common patterns of gene expression. Our work suggests that positional information, especially the relative spacing between transcription factor binding sites, may represent a common organizing principle of transcription control regions.
Journal: Genome Biology, 2003 DOI: 10.1186/gb-2003-4-7-r43 PMID: 12844359.0 PMCID: PMC193630
Preprints
63P. Integrative Multi-omic Profiling of Two Human Decedents Receiving Pig Heart Xenografts Reveals Strong Perturbations in Early Immune-Cell and Cellular Metabolism Responses
Schmauch, Piening, Xia, Zhu, Stern, Zhang, Dowdell, Loza, Mohebnasab, Gragert, Khalil, Camellato, de Oliveira, O'Brien, Weldon, Lin, Gao, Kagermazova, Kim, Loupy, Heguy, Taylor, Zhu, Gao, Divya Gandla, Kriyana Reddy, Andrew Chang, Michael, Jiang, Jian, Narula, Linna-Kuosmanen, Kaikkonen-Maatta, Lorber, Kellis, Tatapudi, Ayares, Griesemer, Mangiola, Pass, Snyder, Montgomery, Boeke, Keating
Background Recent advances in xenotransplantation in living and decedent humans using pig xenografts have laid promising groundwork towards future emergency use and first in human trials. Major obstacles remain though, including a lack of knowledge of the genetic incompatibilities between pig donors and human recipients which may led to harmful immune responses against the xenograft or dysregulation of normal physiology. In 2022 two pig heart xenografts were transplanted into two brain-dead human decedents with a minimized immunosuppression regime, primarily to evaluate onset of hyper-acute antibody mediated rejection and sustained xenograft function over 3 days. Methods We performed multi-omic profiling to assess the dynamic interactions between the pig and human genomes in the first two pig heart-xenografts transplants into human decedents. To assess global and specific biological changes that may correlate with immune-related outcomes and xenograft function, we generated transcriptomic, lipidomic, proteomic and metabolomics datasets, across blood and tissue samples collected every 6 hours over the 3-day procedures. Results Single-cell datasets in the 3-day pig xenograft-decedent models show dynamic immune activation processes. We observe specific scRNA-seq, snRNA-seq and geospatial transcriptomic changes of early immune-activation leading to pronounced downstream T-cell activity and hallmarks of early antibody mediated rejection (AbMR) and/or ischemia reperfusion injury (IRI) in the first xenograft recipient. Using longitudinal multiomic integrative analyses from blood in addition to antigen presentation pathway enrichment, we also observe in the first xeno-heart recipient significant cellular metabolism and liver damage pathway changes that correlate with profound physiological dysfunction whereas, these signals are not present in the other xenograft recipient.
Date: 2023 Jun 5
arXiv: 2023.06.05.543406
62P. Rabies virus-based barcoded neuroanatomy resolved by single-cell RNA and in situ sequencing
Zhang, Jin, Yao, Matsuyama, van Velthoven, Sullivan, Sun, Kellis, Tasic, Tasic, Wickersham, Chen
Mapping the connectivity of diverse neuronal types provides the foundation for understanding the structure and function of neural circuits. High-throughput and low-cost neuroanatomical techniques based on RNA barcode sequencing have the potential to achieve circuit mapping at cellular resolution and a brain-wide scale, but existing Sindbis virus-based techniques can only map long-range projections using anterograde tracing approaches. Rabies virus can complement anterograde tracing approaches by enabling either retrograde labeling of projection neurons or monosynaptic tracing of direct inputs to genetically targeted postsynaptic neurons. However, barcoded rabies virus has so far been only used to map non-neuronal cellular interactions in vivo and synaptic connectivity of cultured neurons. Here we combine barcoded rabies virus with single-cell and in situ sequencing to perform retrograde labeling and transsynaptic labeling in the mouse brain. We sequenced 96 retrogradely labeled cells and 295 transsynaptically labeled cells using single-cell RNAseq, and 4,130 retrogradely labeled cells and 2,914 transsynaptically labeled cells in situ. We determined the transcriptomic identities of rabies virus-infected cells robustly using both single-cell RNA-seq and in situ sequencing. We then distinguished long-range projecting cortical cell types from multiple cortical areas and identified cell types with converging or diverging synaptic connectivity. Combining in situ sequencing with barcoded rabies virus thus complements existing sequencing-based neuroanatomical techniques and provides a potential path for mapping synaptic connectivity of neuronal types at scale.
Date: 2023 Mar 16
arXiv: 2023.03.16.532873
61P. An Autoimmune Transcriptional Circuit Driving Foxp3+ Regulatory T cell Dysfunction
Sumida, Lincoln, He, Park, Ota, Stillwell, Leissa, Fujio, Kulminski, Epstein, Bernstein, Kellis, Hafler
Autoimmune diseases, among the most common disorders of young adults, are mediated by genetic and environmental factors. While CD4+Foxp3+ regulatory T cells (Tregs) play a central role in preventing autoimmunity, the molecular mechanism underlying their dysfunction is unknown. Here, we performed comprehensive transcriptomic and epigenomic profiling of Tregs in the autoimmune disease multiple sclerosis (MS) to identify central transcriptional programs regulating human autoimmunity. We discovered that upregulation of a primate-specific short PRDM1 isoform (PRDM1-S) induces SGK1 independent from evolutionally conserved long PRDM1, leading to destabilization of Foxp3 and Treg dysfunction. This aberrant PRDM1-S/SGK1 axis is shared among other autoimmune diseases. Furthermore, by chromatin landscape profiling in MS Tregs we identified a PRDM1-S specific cis-regulatory element associated with enriched binding of AP-1/IRF transcription factors. Our study identifies evolutionally emerged PRDM1-S and epigenetic priming of AP-1/IRF as key drivers of pathogenic Treg programs leading to human autoimmune disease.
Date: 2022 Dec 2
arXiv: 2022.12.02.518871
60P. QClus: Robust and reliable preprocessing method for human heart snRNA-seq
Schmauch, Ojanen, Galani, Jalkanen, Hollmen, Gunn, Kiviniemi, Kaikkonen-Maatta, Kellis, Linna-Kuosmanen
Single nuclei RNA sequencing (snRNA-seq) is widely used to study tissues and diseases. However, the technique remains challenging, as cytoplasmic RNA often contaminates nuclei-containing droplets and may even complicate the removal of empty droplets. Incomplete removal of contaminated background signal masks cell type-specific signal and interferes with differential gene expression analysis. Thus, removing empty droplets and highly contaminated nuclei is of paramount importance in snRNA-seq analysis. This is especially the case in solid tissues such as the heart. Here, we present QClus, a novel nuclei filtering method targeted to human heart samples. In the human heart, most of the contamination originates from cytoplasmic RNA, stemming from cardiomyocytes. Therefore, we use specific metrics such as splicing, mitochondrial gene expression, nuclear gene expression, and non-cardiomyocyte and cardiomyocyte marker gene expression to cluster nuclei and filter empty and highly contaminated droplets. This approach combined with other filtering steps enables for flexible, automated, and reliable cleaning of samples with varying number of nuclei, quality, and contamination levels. To confirm the robustness of our method, we ran QClus on our dataset of 32 human heart samples and compared results to six alternative methods. None of the methods resulted in the same filtering quality as QClus, each of them failing significantly in some samples. As snRNA-seq is predominantly used on unique human tissue samples, sample failure due to inadequate data processing seems unacceptable. Our study shows that, instead of using universal preprocessing methods, challenging tissue types, such as heart tissue, may benefit from methods that are more specific to the tissue under study, as QClus outperforms the other methods primarily due to improved integration of sample type-specific features.
Date: 2022 Oct 21
arXiv: 2022.10.21.513315
59P. Global landscape of the host response to SARS-CoV-2 variants reveals viral evolutionary trajectories
Bouhaddou, Reuschl, Polacco, Thorne, Ummadi, Ye, Rosales, Pelin, Batra, Jang, Xu, Moen, Richards, Zhou, Harjai, Stevenson, Rojc, Ragazzini, Whelan, Furnon, Lorenzo, Cowton, Syed, Ciling, Deutsch, Pirak, Dowgier, Mesner, Turner, McGovern, Rodriguez, Leiva-Rebollo, Dunham, Zhong, Eckhardt, Fossati, Liotta, Kehrer Cupic, Rutkowska, Mena, Aslam, Hoffert, Foussard, Pham, Lyons, Donahue, Griffin, Nugent, Holden, Deans, Aviles, López-Martín, Jimeno, Obernier, Fabius, Soucheray, Hüttenhain, Jungreis, Kellis, Echeverria, Verba, Bonfanti, Beltrao, Sharan, Doudna, Martinez-Sobrido, Patel, Palmarini, Miorin, White, Swaney, García-Sastre, Jolly, Zuliani-Alvarez, Towers, Krogan
A series of SARS-CoV-2 variants of concern (VOCs) have evolved in humans during the COVID-19 pandemic—Alpha, Beta, Gamma, Delta, and Omicron. Here, we used global proteomic and genomic analyses during infection to understand the molecular responses driving VOC evolution. We discovered VOC-specific differences in viral RNA and protein expression levels, including for N, Orf6, and Orf9b, and pinpointed several viral mutations responsible. An analysis of the host response to VOC infection and comprehensive interrogation of altered virus-host protein-protein interactions revealed conserved and divergent regulation of biological pathways. For example, regulation of host translation was highly conserved, consistent with suppression of VOC replication in mice using the translation inhibitor plitidepsin. Conversely, modulation of the host inflammatory response was most divergent, where we found Alpha and Beta, but not Omicron BA.1, antagonized interferon stimulated genes (ISGs), a phenotype that correlated with differing levels of Orf6. Additionally, Delta more strongly upregulated proinflammatory genes compared to other VOCs. Systematic comparison of Omicron subvariants revealed BA.5 to have evolved enhanced ISG and proinflammatory gene suppression that similarly correlated with Orf6 expression, effects not seen in BA.4 due to a mutation that disrupts the Orf6-nuclear pore interaction. Our findings describe how VOCs have evolved to fine-tune viral protein expression and protein-protein interactions to evade both innate and adaptive immune responses, offering a likely explanation for increased transmission in humans.
Date: 2022 Oct 19
arXiv: 2022.10.19.512927
58P. Enhancer Reprogramming in Melanoma Immune Checkpoint Therapy Resistance
Maitituoheti, Shi, Tang, Ho, Terranova, Galani, Keung, Creasy, Wu, Chen, Chen, Singh, Chaudhri, Anvar, Tarantino, Yang, Sarkar, Jiang, Malke, Haydu, Burton, Davies, Gershenwald, Hwu, Lazar, Cheah, Soule, Levine, Bernatchez, Saladi, Liu, Wargo, Boland, Kellis, Rai
Immune checkpoint blockade (ICB) therapy has improved long-term survival for patients with advanced melanoma. However, there is critical need to identify potential biomarkers of response and actionable strategies to improve response rates. Through generation and analysis of 148 chromatin modification maps for 36 melanoma samples from patients treated with anti-PD- 1, we identified significant enrichment of active enhancer states in non-responders at baseline. Analysis of an independent cohort of 20 samples identified a set of 437 enhancers that predicted response to anti-PD-1 therapy (Area Under the Curve of 0.8417). The activated non-responder enhancers marked a group of key regulators of several pathways in melanoma cells (including c- MET, TGFβ, EMT and AKT) that are known to mediate resistance to ICB therapy and several checkpoint receptors in T cells. Epigenetic editing experiments implicated involvement of c-MET enhancers in the modulation of immune response. Finally, inhibition of enhancers and repression of these pathways using bromodomain inhibitors along with anti-PD-1 therapy significantly decreased melanoma tumor burden and increased T-cell infiltration. Together, these findings identify a potential enhancer-based biomarker of resistance to anti-PD-1 and suggest enhancer blockade in combination with ICB as a potential strategy to improve responses.
Date: 2022 Aug 31
arXiv: 2022.08.31.506051
57P. Exercise Training Remodels Inguinal White Adipose Tissue Through Adaptations in Innervation, Vascularization and the Extracellular Matrix
Nigro, Vamvini, Yang, Caputo, Ho, Papadopoulos, Carbone, Conlin, He, Hirshman, White, Robidoux, Hickner, Nielsen, Pedersen, Kellis, Middelbeek, Goodyear
Inguinal white adipose tissue (iWAT) is essential for the beneficial effects of exercise training on metabolic health. Extracellular matrix (ECM) composition, innervation, and vascularization are all important regulators of iWAT function, yet whether exercise training improves these structural components of iWAT is unknown. Using biochemical, imaging, and multi-omics analyses we find that 11-days of wheel running in male mice causes profound iWAT remodeling including decreased ECM deposition and increased vascularization and innervation. We identify adipose stem cells as the main contributors to training-induced ECM remodeling, determine that training causes a shift from hypertrophic to insulin-sensitive adipocyte subpopulations, show that the PRDM16 transcriptional complex is necessary for iWAT remodeling and beiging, and discover neuronal growth regulator 1 (NEGR1) as a link between PRDM16 and neuritogenesis. Exercise training leads to remarkable adaptations to iWAT structure and cell-type composition that can confer beneficial changes in tissue metabolism.
Date: 2022 Aug 9
arXiv: 2022.08.09.503375
56P. The ENCODE Imputation Challenge: A critical assessment of methods for cross-cell type imputation of epigenomic profiles
Schreiber, Boix, Lee, Li, Guan, Chang, Chang, Hawkins-Hooker, Schölkopf, Schweikert, Carulla, Canakoglu, Guzzo, Nanni, Masseroli, Carman, Pinoli, Hong, Yip, Spence, Batra, Song, Mahony, Zhang, Tan, Shen, Sun, Shi, Adrian, Sandstrom, Farrell, Halow, Lee, Jiang, Yang, Epstein, Strattan, Snyder, Kellis, Noble, Kundaje, ENCODE Imputation Challenge Participants
Functional genomics experiments are invaluable for understanding mechanisms of gene regulation. However, comprehensively performing all such experiments, even across a fixed set of sample and assay types, is often infeasible in practice. A promising alternative to performing experiments exhaustively is to, instead, perform a core set of experiments and subsequently use machine learning methods to impute the remaining experiments. However, questions remain as to the quality of the imputations, the best approaches for performing imputations, and even what performance measures meaningfully evaluate performance of such models. In this work, we address these questions by comprehensively analyzing imputations from 23 imputation models submitted to the ENCODE Imputation Challenge. We find that measuring the quality of imputations is significantly more challenging than reported in the literature, and is confounded by three factors: major distributional shifts that arise because of differences in data collection and processing over time, the amount of available data per cell type, and redundancy among performance measures. Our systematic analyses suggest several steps that are necessary, but also simple, for fairly evaluating the performance of such models, as well as promising directions for more robust research in this area.
Date: 2022 Jul 30
arXiv: 2022.07.30.502157
55P. Huntington's Disease Produces Multiplexed Transcriptional Vulnerabilities of Striatal D1-D2 and Striosome-Matrix Neurons
Matsushima, Pineda,Crittenden, Lee, Galani, Mantero, Kellis, Heiman, Graybiel
Striatal cell-type-specific vulnerability in Huntington's disease (HD) preferentially affects dopamine D2R-expressing projection neurons (SPNs), compatible with manifest motor symptomatology in HD. Transcriptional studies of striatal striosome-matrix compartmentalization in HD are, however, limited, despite pathologic evidence for striosome vulnerability aligning with early mood symptomatology. We used single-nucleus RNA-sequencing on striatal samples from two murine models, and rare Grade 1 HD patient tissues, to examine striosome and matrix sub-clusters within parent D1 and D2 SPN clusters. In human HD, striosomal SPNs were the most depleted SPN population. Surprisingly, for both mouse models, transcriptomic distinctiveness was diminished more for striosome-matrix SPNs than for D1-D2 SPNs. Compartmental markers were dysregulated so as to cancel endogenous identities as striosomal or matrix SPNs, but markers for D1-D2 exhibited less identity obscuring. The canonical striosome-matrix as well as D1-D2 organizations of the striatum thus are both strongly, but differentially, compromised in HD and are targets for therapeutics.
Date: 2022 Apr 25
arXiv: 2022.04.25.489455
54P. Single-cell mosaicism analysis reveals cell-type-specific somatic mutational burden in Alzheimer's Dementia
Kousi, Boix, Park, Mathys, Sledzieski, Peng, Bennett, Tsai, Kellis
Despite significant advances in identifying genetic drivers of neurodegenerative disorders, the majority of affected individuals lack molecular genetic diagnosis, with somatic mutations proposed as one potential contributor to increased risk. Here, we report the first cell-type-specific map of somatic mosaicism in Alzheimer's Dementia (AlzD), using 4,014 cells from prefrontal cortex samples of 19 AlzD and 17 non-AlzD individuals. We integrate full-transcript single-nucleus RNA-seq (SMART-Seq) with matched individual-level whole-genome sequencing to jointly infer mutational events and the cell-type in which they occurred. AlzD individuals show increased mutational burden, localized in excitatory neurons, oligodendrocytes, astrocytes and disease-associated “senescent” cells. High-mutational-burden cells showed mutational enrichment and similar single-cell expression profiles in AlzD cases versus non-AlzD individuals, indicating cellular-level genotype-to-phenotype correlation. Somatic mutations are specifically enriched for known AlzD genes, and implicate biologically meaningful cell-type specific processes, including: neuronal energy regulation, endocytic trafficking (NEFM), lipid metabolism (CNP, CRYAB), proteostasis (USP34), cytoskeleton, and microtubule dynamics (MACF1).
Date: 2022 Apr 21
arXiv: 2022.04.21.489103
53P. A partnership of the lipid scramblase XK and of the lipid transfer protein VPS13A at the plasma membrane
Samander, Wu, Pineda, García, Eisen, Leonzino, Uğur, Kellis, Heiman, De Camilli
Chorea-acanthocytosis and McLeod syndrome are diseases with shared clinical manifestations caused by mutations in VPS13A and XK, respectively. Key features of these conditions are the degeneration of caudate neurons and the presence of abnormally shaped erythrocytes. XK belongs to a family of plasma membrane (PM) lipid scramblases whose action results in exposure of PtdSer at the cell surface. VPS13A is an ER-anchored lipid transfer protein with a putative role in the transport of lipids at contacts of the ER with other membranes. Recently VPS13A and XK were reported to interact by still unknown mechanisms. So far, however, there is no evidence for a colocalization of the two proteins at contacts of the ER with the PM, where XK resides, as VPS13A was shown to be localized at contacts between the ER and either mitochondria or lipid droplets. Here we show that VPS13A can also localize at ER-PM contacts via the binding of its PH domain to a cytosolic loop of XK, that such interaction is regulated by an intramolecular interaction within XK and that both VPS13A and XK are highly expressed in the caudate neurons. Binding of the PH domain of VPS13A to XK is competitive with its binding to intracellular membranes that mediate other tethering functions of VPS13A. Our findings support a model according to which VPS13A-dependent lipid transfer between the ER and the PM is coupled to lipid scrambling within the PM. They raise the possibility that defective cell surface exposure of PtdSer may be responsible for neurodegeneration.
Date: 2022 Mar 30
arXiv: 2022.03.30.486314
52P. Single-cell multi-region dissection of brain vasculature in Alzheimer's Disease
Sun, Anne Akay, Murdock, Park, Bubnys, Galani, Mathys, Jiang, Ng, Bennett, Tsai, Kellis
Cerebrovascular breakdown occurs early in Alzheimer's Disease (AD), but its cell-type-specific molecular basis remains uncharacterized. Here, we characterize single-cell transcriptomic differences in human cerebrovasculature across 220 AD and 208 control individuals and across 6 brain regions. We annotate 22,514 cerebrovascular cells in 11 subtypes of endothelial, pericyte, smooth muscle, perivascular fibroblast, and ependymal cells, and how they differ in abundance and gene expression between brain regions. We identify 2,676 AD-differential genes, including lower expression of PDGFRB in pericytes, and ABCB1 and ATP10A in endothelial cells. These AD-differential genes reveal common upstream regulators, including MECOM, EP300, and KLF4, whose targeting may help restore vasculature function. We find coordinated vasculature-glial-neuronal co-expressed gene modules supported by ligand-receptor pairs, involved in axon growth/degeneration and neurogenesis, suggesting mechanistic mediators of neurovascular unit dysregulation in AD. Integration with AD genetics reveals 125 AD-differential genes directly linked to AD-associated genetic variants (through vasculature-specific eQTLs, Hi-C, and correlation-based evidence), 559 targeted by AD-associated regulators, and 661 targeted by AD-associated ligand-receptor signaling. Lastly, we show that APOE4-genotype associated differences are significantly enriched among AD-associated genes in capillary and venule endothelial cells, and subsets of pericytes and fibroblasts, which underlie the vascular dysregulation in APOE4-associated cognitive decline. Overall, our multi-region molecular atlas of differential human cerebrovasculature genes and pathways in AD can help guide early-stage AD therapeutics.
Date: 2022 Feb 9
arXiv: 2022.02.09.479797
51P. Neurons burdened by DNA double strand breaks incite microglia activation through antiviral-like signaling in neurodegeneration
Welch, Boix, Schmauch, Davila-Velderrain, Victor, Dileep, Bozzelli, Su, Cheng, Lee, Leary, Pfenning, Kellis, Tsai
DNA double strand breaks (DSBs) are linked to aging, neurodegeneration, and senescence1,2. However, the role played by neurons burdened with DSBs in disease-associated neuroinflammation is not well understood. Here, we isolate neurons harboring DSBs from the CK-p25 mouse model of neurodegeneration through fluorescence-activated nuclei sorting (FANS), and characterize their transcriptomes using single-nucleus, bulk, and spatial sequencing techniques. We find that neurons harboring DSBs enter a late-stage DNA damage response marked by the activation of senescent and antiviral-like immune pathways. We identify the NFkB transcription factor as a master regulator of immune gene expression in DSB-bearing neurons, and find that the expression of cytokines like Cxcl10 and Ccl2 develop in DSB-bearing neurons before glial cell types. Alzheimer's Disease pathology is significantly associated with immune activation in excitatory neurons, and direct purification of DSB-bearing neurons from Alzheimer's Disease brain tissue further validates immune gene upregulation. Spatial transcriptomics reveal that regions of brain tissue dense with DSB-bearing neurons also harbor signatures of inflammatory microglia, which is ameliorated by NFkB knock down in neurons. Inhibition of NFkB or depletion of Ccl2 and Cxcl10 in DSB-bearing neurons also reduces microglial activation in organotypic brain slice culture. In conclusion, we find that in the context of age-associated neurodegenerative disease, DSBs activate immune pathways in neurons, which in turn adopt a senescence associated secretory phenotype to elicit microglia activation. These findings highlight a novel role for neurons in the mechanism of age-associated neuroinflammation.
Date: 2021 Dec 23
arXiv: 2021.12.23.474002
50P. isomiRs-specific differential expression is the rule, not the exception: Are we missing hundreds of species in microRNA analysis?
Schmauch, Laitinen, Turunen, Väänänen, Malm, Kellis, Kaikkonen, Linna-Kuosmanen
MicroRNAs (miRNAs) are small RNA molecules that act as regulators of gene expression through targeted mRNA degradation. They are involved in many biological and pathophysiological processes and are widely studied as potential biomarkers and therapeutics agents for human diseases, including cardiovascular disorders. Recently discovered isoforms of miRNAs (isomiRs) exist in high quantities and are very diverse. Despite having few differences with their corresponding reference miRNAs, they display specific functions and expression profiles, across tissues and conditions. However, they are still overlooked and understudied, as we lack a comprehensive view on their condition-specific regulation and impact on differential expression analysis. Here, we show that isomiRs can have major effects on differential expression analysis results, as their expression is independent of their host miRNA genes or reference sequences. We present two miRNA-seq datasets from human umbilical vein endothelial cells, and assess isomiR expression in response to senescence and compartment-specificity (nuclear/cytosolic) under hypoxia. We compare three different methods for miRNA analysis, including isomiR-specific analysis, and show that ignoring isomiRs induces major biases in differential expression. Moreover, isomiR analysis permits higher resolution of complex signal dissection, such as the impact of hypoxia on compartment localization, and differential isomiR type enrichments between compartments. Finally, we show important distribution differences across conditions, independently of global miRNA expression signals. Our results raise concerns over the quasi exclusive use of miRNA reference sequences in miRNA-seq processing and experimental assays. We hope that our work will guide future isomiR expression studies, which will correct some biases introduced by golden standard analysis, improving the resolution of such assays and the biological significance of their downstream studies.
Date: 2021 Dec 15
arXiv: 2021.12.15.472814
49P. Single-cell dissection of obesity-exercise axis in adipose-muscle tissues
Yang, Vamvini, Nigro,Ho, Galani, Alvarez, Tanigawa, Laakso, Agudelo, Pajukanta, Middelbeek, Grove, Goodyear, Kellis
Regular physical exercise has long been recognized to reverse the effects of diet-induced obesity, but the molecular mechanisms mediating these multi-tissue beneficial effects remain uncharacterized. Here, we address this challenge by studying the opposing effects of exercise training and high-fat diet at single-cell, deconvolution and tissue-level resolutions across 3 metabolic tissues. We profile scRNA-seq in 204,883 cells, grouped into 53 distinct cell subtypes/states in 22 major cell types, from subcuta-neous and visceral white adipose tissue (WAT), and skeletal muscle (SkM) in mice with diet and exercise training interventions. With a great number of mesenchymal stem cells (MSCs) profiled, we compared depot-specific adipose stem cell (ASC) states, and defined 7 distinct fibro-adipogenic progenitor (FAP) states in SkM including discovering and validating a novel CD140+/CD34+/SCA1-FAP population. Exercise- and obesity-regulated proportion, transcriptional and cell-cell interaction changes were most strongly pronounced in and centered around ASCs, FAPs, macrophages and T-cells. These changes reflected thermogenesis-vs-lipogenesis and hyperplasia-vs-hypertrophy shifts, clustered in pathways including extracellular matrix remodeling and circadian rhythm, and implicated complex single- and multi-tissue communication including training-associated shift of a cytokine from binding to its decoy receptor on ASCs to true receptor on M2 macrophages in vWAT. Overall, our work provides new insights on the metabolic protective effects of exercise training, uncovers a previously-underappreciated role of MSCs in mediating tissue-specific and multi-tissue effects, and serves as a model for multitissue single-cell analyses in physiologically complex and multifactorial traits exemplified by obesity and exercise training.
Date: 2021 Nov 22
arXiv: 2021.11.22.469622
48P. Metabolic resilience is encoded in genome plasticity
Agudelo, Tuyeras, Llinares, Morcuende, Park, Sun, Linna-Kuosmanen, Atabaki-Pasdar, Ho, Galani, Franks, Kutlu, Grove, Femenia, Kellis
Metabolism plays a central role in evolution, as resource conservation is a selective pressure for fitness and survival. Resource-driven adaptations offer a good model to study evolutionary innovation more broadly. It remains unknown how resource-driven optimization of genome function integrates chromatin architecture with transcriptional phase transitions. Here we show that tuning of genome architecture and heterotypic transcriptional condensates mediate resilience to nutrient limitation. Network genomic integration of phenotypic, structural, and functional relationships reveals that fat tissue promotes organismal adaptations through metabolic acceleration chromatin domains and heterotypic PGC1A condensates. We find evolutionary adaptations in several dimensions; low conservation of amino acid residues within protein disorder regions, nonrandom chromatin location of metabolic acceleration domains, condensate-chromatin stability through cis-regulatory anchoring and encoding of genome plasticity in radial chromatin organization. We show that environmental tuning of these adaptations leads to fasting endurance, through efficient nuclear compartmentalization of lipid metabolic regions, and, locally, human-specific burst kinetics of lipid cycling genes. This process reduces oxidative stress, and fatty-acid mediated cellular acidification, enabling endurance of condensate chromatin conformations. Comparative genomics of genetic and diet perturbations reveal mammalian convergence of phenotype and structural relationships, along with loss of transcriptional control by diet-induced obesity. Further, we find that radial transcriptional organization is encoded in functional divergence of metabolic disease variant-hubs, heterotypic condensate composition, and protein residues sensing metabolic variation. During fuel restriction, these features license the formation of large heterotypic condensates that buffer proton excess, and shift viscoelasticity for condensate endurance. This mechanism maintains physiological pH, reduces pH-resilient inflammatory gene programs, and enables genome plasticity through transcriptionally driven cell-specific chromatin contacts. In vivo manipulation of this circuit promotes fasting-like adaptations with heterotypic nuclear compartments, metabolic and cell-specific homeostasis. In sum, we uncover here a general principle by which transcription uses environmental fluctuations for genome function, and demonstrate how resource conservation optimizes transcriptional self-organization through robust feedback integrators, highlighting obesity as an inhibitor of genome plasticity relevant for many diseases.
Date: 2021 Jun 25
arXiv: 2021.06.25.449953
47P. Single-cell profiling of the human primary motor cortex in ALS and FTLD
Pineda, Lee, Fitzwalter, Mohammadi, Pregent, Gardashli, Mantero, Engelberg-Cook, DeJesus-Hernandez, van Blitterswijk, Pottier, Rademakers, Oskarsson, Shah, Petersen, Graff-Radford, Boeve, Knopman, Josephs, DeTure, Murray, Dickson, Heiman, Belzil, Kellis
Amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) are two devastating and fatal neurodegenerative conditions. While distinct, they share many clinical, genetic, and pathological characteristics1, and both show selective vulnerability of layer 5b extratelencephalic-projecting cortical populations, including Betz cells in ALS2,3 and von Economo neurons (VENs) in FTLD4,5. Here, we report the first high resolution single-cell atlas of the human primary motor cortex (MCX) and its transcriptional alterations in ALS and FTLD across ~380,000 nuclei from 64 individuals, including 17 control samples and 47 sporadic and C9orf72-associated ALS and FTLD patient samples. We identify 46 transcriptionally distinct cellular subtypes including two Betz-cell subtypes, and we observe a previously unappreciated molecular similarity between Betz cells and VENs of the prefrontal cortex (PFC) and frontal insula. Many of the dysregulated genes and pathways are shared across excitatory neurons, including stress response, ribosome function, oxidative phosphorylation, synaptic vesicle cycle, endoplasmic reticulum protein processing, and autophagy. Betz cells and SCN4B+ long-range projecting L3/L5 cells are the most transcriptionally affected in both ALS and FTLD. Lastly, we find that the VEN/Betz cell-enriched transcription factor, POU3F1, has altered subcellular localization, co-localizes with TDP-43 aggregates, and may represent a cell type-specific vulnerability factor in the Betz cells of ALS and FTLD patient tissues.
Date: 2021 Jul 7
arXiv: 2021.07.07.451374
46P. Single-cell anatomical analysis of human hippocampus and entorhinal cortex uncovers early-stage molecular pathology in Alzheimer's disease
Davila-Velderrain, Mathys, Mohammadi, Ruzicka, Jiang, Ng, Bennett,Tsai, Kellis
The human hippocampal formation plays a central role in Alzheimer's disease (AD) progression, cognitive traits, and the onset of dementia; yet its molecular states in AD remain uncharacterized. Here, we report a comprehensive single-cell transcriptomic dissection of the human hippocampus and entorhinal cortex across 489,558 cells from 65 individuals with varying stages of AD pathology. We transcriptionally characterize major brain cell types and neuronal classes, including 17 glutamatergic and 8 GABAergic neuron subpopulations. Combining evidence from human and mouse tissue-microdissection, neuronal cell isolation and spatial transcriptomics, we show that single-cell expression patterns capture fine-resolution neuronal anatomical topography. By stratifying subjects into early and late pathology groups, we uncover stage-dependent and cell-type specific transcriptional modules altered during AD progression. These include early-stage cell-type specific dysregulation of cellular and cholesterol metabolism, late-stage neuron-glia alterations in neurotransmission, and late-stage signatures of cellular stress, apoptosis, and DNA damage broadly shared across cell types. Late-stage signatures show signs of convergence in hippocampal and cortical cells, while early changes diverge; highlighting the relevance of characterizing molecular pathology across brain regions and AD progression. Finally, we characterize neuron subregion-specific responses to AD pathology and show that CA1 pyramidal neurons are the most transcriptionally altered while CA3 and dentate gyrus granule neurons the least. Our study provides a valuable resource to extend cell type-specific studies of AD to clinically relevant brain regions affected early by pathology in disease progression.
Date: 2021 Jul 1
arXiv: 2021.07.01.450715
45P. Cellular intelligence: dynamic specialization through non-equilibrium multi-scale compartmentalization
Tuyeras, Agudelo, Ram, Loon, Kutlu, Grove, Kellis
Intelligence is usually associated with the ability to perceive, retain and use information to adapt to changes in one's environment. In this context, systems of living cells can be thought of as intelligent entities. Here, we show that the concepts of non-equilibrium tuning and compartmentalization are sufficient to model manifestations of cellular intelligence such as specialization, division, fusion and communication using the language of operads. We implement our framework as an unsupervised learning algorithm, IntCyt, which we show is able to memorize, organize and abstract reference machine-learning datasets through generative and self-supervised tasks. Overall, our learning framework captures emergent properties programmed in living systems, and provides a powerful new approach for data mining.
Date: 2021 Jun 25
arXiv: 2021.06.25.449951
44P. Cardiovascular disease causes proinflammatory microvascular changes in the human right atrium
Linna-Kuosmanen, Schmauch, Galani, Ojanen, Boix, Örd, Toropainen, Singha, Moreau, Harju, Blazeski, Segerstolpe, Lahtinen, Hou, Kang, Meibalan, Agudelo, Kokki, Halonen, Jalkanen, Gunn, MacRae, Hollmén, Hartikainen, Kaikkonen, García-Cardeña, Tavi, Kiviniemi, Kellis
Ischemic heart disease is globally the leading cause of death. It plays a central role in the electrical and structural remodeling of the right atrium, predisposing to arrhythmias, heart failure, and sudden death. Here, we provide the first dissection of the gene expression changes in the live right atrial tissue, using single-nuclei RNA-seq and spatial transcriptomics. We investigate matched samples of the tissue and pericardial fluid and reveal substantial differences in disease- associated gene expression in all cell types, leading to inflammatory microvascular dysfunction and changes in the tissue composition. Our study demonstrates the importance of creating high- resolution cellular maps and partitioning disease signals beyond epicardial coronary arteries and ischemic left ventricle to identify candidate mechanisms leading to more severe types of human cardiovascular disease.
Date: 2021 Jun 23
arXiv: 2021.06.23.449672
43P. Comparison of human and mouse tissues with focus on genes with no 1-to-1 homology
Jeong, Kellis
We assembled a panel of 28 tissue pairs of human and mouse with RNA-Seq data on gene expression. We focused on genes with no 1-to-1 homology, because they pose special challenges. In this way, we identified expression patterns that identify and explain differences between the two species and suggest target genes for therapeutic applications. Here we mention three examples. One pattern is observed by defining the aggregate expression of immunoglobulin genes (which have no homology) as a measure of different levels of an immune response. In Lung, we used this statistic to find genes that have significantly higher expression in low/moderate response, and thus they may be therapy targets: increasing their expression or mimicking their function with medications may help in recovery from inflammation in the lungs. Some of the observed associations are common to human and mouse; other associations involve genes involved in cell-to-cell signaling or in regeneration but were not known to be important in Lung. Second pattern is that in the Small Intestine, mouse expresses much less antimicrobial defensins, while it has much higher expression of enzymes that are found to improve adaptive immune response. Such enzymes may be tested if they improve probiotic supplements that help in gut inflammation and other diseases. Another pattern involves a many-to-many homology group of defensins that did not have a described function. In human tissues, expression of its genes was found only in a study of a disease of hair covered skin, but several of its genes are highly expressed in two tissues of our panel: mouse Skin and to a lesser degree mouse Vagina. This suggests that those genes or their homologs in other species may provide non-antibiotic medications for hair covered skin and other tissues with microbiome that includes fungi.
Date: 2021 May 23
arXiv: 2021.05.22.445250
42P. Leveraging single-cell ATAC-seq to identify disease-critical fetal and adult brain cell types
Kim, Jagadeesh, Dey, Shen, Raychaudhuri, Kellis, Price
Prioritizing disease-critical cell types by integrating genome-wide association studies (GWAS) with functional data is a fundamental goal. Single-cell chromatin accessibility (scATAC-seq) and gene expression (scRNA-seq) have characterized cell types at high resolution, and early work on integrating GWAS with scRNA-seq has shown promise, but work on integrating GWAS with scATAC-seq has been limited. Here, we identify disease-critical fetal and adult brain cell types by integrating GWAS summary statistics from 28 brain-related diseases and traits (average N =298K) with 3.2 million scATAC-seq and scRNA-seq profiles from 83 cell types. We identified disease-critical fetal (resp. adult) brain cell types for 22 (resp. 23) of 28 traits using scATAC-seq data, and for 8 (resp. 17) of 28 traits using scRNA-seq data. Notable findings using scATAC-seq data included highly significant enrichments of fetal photoreceptor cells for major depressive disorder, fetal ganglion cells for BMI, fetal astrocytes for ADHD, and adult VGLUT2 excitatory neurons for schizophrenia. Our findings improve our understanding of brain-related diseases and traits, and inform future analyses of other diseases/traits.
Date: 2021 May 21
arXiv: 2021.05.20.445067
41P. Single-cell dissection of the human cerebrovasculature in health and disease
Garcia, Sun, Lee, Godlewski, Galani, Mantero, Bennett, Sahin, Kellis, Heiman
Despite the importance of the blood-brain barrier in maintaining normal brain physiology and in understanding neurodegeneration and CNS drug delivery, human cerebrovascular cells remain poorly characterized due to their sparsity and dispersion. Here, we perform the first single-cell characterization of the human cerebrovasculature using both ex vivo fresh-tissue experimental enrichment and post mortem in silico sorting of human cortical tissue samples. We capture 31,812 cerebrovascular cells across 17 subtypes, including three distinct subtypes of perivascular fibroblasts as well as vasculature-coupled neurons and glia. We uncover human-specific expression patterns along the arteriovenous axis and determine previously uncharacterized cell type-specific markers. We use our newly discovered human-specific signatures to study changes in 3,945 cerebrovascular cells of Huntington's disease patients, which reveal an activation of innate immune signaling in vascular and vasculature-coupled cell types and the concomitant reduction to proteins critical for maintenance of BBB integrity. Finally, our study provides a comprehensive resource molecular atlas of the human cerebrovasculature to guide future biological and therapeutic studies.
Date: 2021 Apr 27
arXiv: 2021.04.26.440975
40P. Structural variant selection for high-altitude adaptation using single-molecule long-read sequencing
Shi, Jia, Zhao, Sun, Liang, Park, Zhao, Wang, Chen, Song, Yu, Jia, Wang, Xiao, Liu, Wu, Zhong, Wu, Cui, Bo, Wu, Kellis, He
Structural variants (SVs) can be important drivers of human adaptation with strong effects, but previous studies have focused primarily on common variants with weak effects. Here, we used large-scale single-molecule long-read sequencing of 320 Tibetan and Han samples, to show that SVs are key drivers of selection under high-altitude adaptation. We expand the landscape of global SVs, apply robust models of selection and population differentiation combining SVs, SNPs and InDels, and use epigenomic analyses to predict driver enhancers, target genes, upstream regulators, and biological functions, which we validate using enhancer reporter and DNA pull-down assays. We reveal diverse Tibetan-specific SVs affecting the cis- and trans-regulatory circuitry of diverse biological functions, including hypoxia response, energy metabolism, lung function, etc. Our study greatly expands the global SV landscape, reveals the central role of gene-regulatory circuitry rewiring in human adaptation, and illustrates the diverse functional roles that SVs can play in human biology.
Date: 2021 Mar 30
arXiv: 2021.03.27.436702
39P. Single-cell deconvolution of 3,000 post-mortem brain samples for eQTL and GWAS dissection in mental disorders
Park, He, Davila-Velderrain, Hou, Mohammadi, Mathys, Peng, Bennett, Tsai, Kellis
Thousands of genetic variants acting in multiple cell types underlie complex disorders, yet most gene expression studies profile only bulk tissues, making it hard to resolve where genetic and non-genetic contributors act. This is particularly important for psychiatric and neurodegenerative disorders that impact multiple brain cell types with highly-distinct gene expression patterns and proportions. To address this challenge, we develop a new framework, SPLITR, that integrates single-nucleus and bulk RNA-seq data, enabling phenotype-aware deconvolution and correcting for systematic discrepancies between bulk and single-cell data. We deconvolved 3,387 post-mortem brain samples across 1,127 individuals and in multiple brain regions. We find that cell proportion varies across brain regions, individuals, disease status, and genotype, including genetic variants in TMEM106B that impact inhibitory neuron fraction and 4,757 cell-type-specific eQTLs. Our results demonstrate the power of jointly analyzing bulk and single-cell RNA-seq to provide insights into cell-type-specific mechanisms for complex brain disorders.
Date: 2021 Jan 21
arXiv: 2021.01.21.426000
38P. Conserved epigenetic regulatory logic infers genes governing cell identity
Shim, Sinniah, Xu, Vitrinel, Alexanian, Andreoletti, Shen, Sun, Balderson, Boix, Peng, Jing, Wang, Kellis, Tam, Smith, Piper, Christiaen, Nguyen, Bodén, Palpant
Determining genes orchestrating cell differentiation in development and disease remains a fundamental goal of cell biology. This study establishes a genome-wide metric based on the gene-repressive tri-methylation of histone 3 lysine 27 (H3K27me3) across hundreds of diverse cell types to identify genetic regulators of cell differentiation. We introduce a computational method, TRIAGE, that uses discordance between gene-repressive tendency and expression to identify genetic drivers of cell identity. We apply TRIAGE to millions of genome-wide single-cell transcriptomes, diverse omics platforms, and eukaryotic cells and tissue types. Using a wide range of data, we validate TRIAGE's performance for identifying cell-type specific regulatory factors across diverse species including human, mouse, boar, bird, fish, and tunicate. Using CRISPR gene editing, we use TRIAGE to experimentally validate RNF220 as a regulator of Ciona cardiopharyngeal development and SIX3 as required for differentiation of endoderm in human pluripotent stem cells. A record of this paper's Transparent Peer Review process is included in the Supplemental Information.
Date: 2020 Oct 9
bioRxiv: 635516.0
37P. Exome-wide age-of-onset analysis reveals exonic variants in ERN1, TACR3 and SPPL2C associated with Alzheimer's disease
He, Loika, Park, Genotype Tissue Expression (GTEx) consortium, Bennett, Kellis, Kulminski, for the Alzheimer's Disease Neuroimaging Initiative
Despite recent discovery in GWAS of genomic variants associated with Alzheimer's disease (AD), its underlying biological mechanisms are still elusive. Discovery of novel AD-associated genetic variants, particularly in coding regions and from APOE ε4 non-carriers, is critical for understanding the pathology of AD. In this study, we carried out an exome-wide association analysis of age-of-onset of AD with ~20,000 subjects and placed more emphasis on APOE ε4 non-carriers. Using Cox mixed-effects models, we find that age-of-onset shows a stronger genetic signal than AD case-control status, capturing many known variants with stronger significance, and also revealing new variants. We identified two novel rare variants, rs56201815, a synonymous variant in ERN1, from the analysis of APOE ε4 non-carriers, and a missense variant rs144292455 in TACR3. In addition, we detected rs12373123, a common missense variant in SPPL2C in the MAPT region in APOE ε4 non-carriers. In an attempt to unravel their regulatory and biological functions, we found that the minor allele of rs56201815 was associated with lower average FDG uptake across five brain regions in ADNI. Our eQTL analyses based on 6198 gene expression samples from ROSMAP and GTEx revealed that the minor allele of rs56201815 was associated with elevated expression of ERN1, a key gene triggering unfolded protein response (UPR), in multiple brain regions, including posterior cingulate cortex and nucleus accumbens. Our cell-type-specific eQTL analysis of based on ~80,000 single nuclei in the prefrontal cortex revealed that the protective minor allele of rs12373123 significantly increased expression of GRN in microglia, and was associated with MAPT expression in astrocytes. These findings provide novel evidence supporting the hypothesis of the potential involvement of the UPR to ER stress in the pathological pathway of AD, and also give more insights into underlying regulatory mechanisms behind the pleiotropic effects of rs12373123 in multiple degenerative diseases including AD and Parkinson's disease.
Date: 2020 Jun 27
arXiv: 2020.01.28.923789
36P. SARS-CoV-2 gene content and COVID-19 mutation impact by comparing 44 Sarbecovirus genomes
Jungreis, Sealfon, Kellis
Despite its overwhelming clinical importance, the SARS-CoV-2 gene set remains unresolved, hindering dissection of COVID-19 biology. Here, we use comparative genomics to provide a high-confidence protein-coding gene set, characterize protein-level and nucleotide-level evolutionary constraint, and prioritize functional mutations from the ongoing COVID-19 pandemic. We select 44 complete Sarbecovirus genomes at evolutionary distances ideally-suited for protein-coding and non-coding element identification, create whole-genome alignments, and quantify protein-coding evolutionary signatures and overlapping constraint. We find strong protein-coding signatures for all named genes and for 3a, 6, 7a, 7b, 8, 9b, and also ORF3c, a novel alternate-frame gene. By contrast, ORF10, and overlapping-ORFs 9c, 3b, and 3d lack protein-coding signatures or convincing experimental evidence and are not protein-coding. Furthermore, we show no other protein-coding genes remain to be discovered. Cross-strain and within-strain evolutionary pressures largely agree at the gene, amino-acid, and nucleotide levels, with some notable exceptions, including fewer-than-expected mutations in nsp3 and Spike subunit S1, and more-than-expected mutations in Nucleocapsid. The latter also shows a cluster of amino-acid-changing variants in otherwise-conserved residues in a predicted B-cell epitope, which may indicate positive selection for immune avoidance. Several Spike-protein mutations, including D614G, which has been associated with increased transmission, disrupt otherwise-perfectly-conserved amino acids, and could be novel adaptations to human hosts. The resulting high-confidence gene set and evolutionary-history annotations provide valuable resources and insights on COVID-19 biology, mutations, and evolution.
Date: 2020 Sep 2
arXiv: 2020.06.02.130955
35P. Genus-wide characterization of bumblebee genomes reveals variation associated with key ecological and behavioral traits of pollinators
Sun, Huang, Wang, Zhao, Su, Thomas, Zhao, Zhang, Jungreis, Kellis, Vicario, Sharakhov, Bondarenko, Hasselmann, Kim, Paten, Penso-Dolfin, Wang, Chang, Gao, Ma, Ma, Zhang, Zhang, Zhang, Ruzzante, Robertson, Zhu, Liu, Yang, Ding, Wang, Xu, Liang, Itgen, Mee, Sadd, Cao, Zhang, Hahn, Schaack, Barribeau, Williams, Waterhouse, Mueller
Bumblebees are a diverse group of globally important pollinators in natural ecosystems and for agricultural food production. With both eusocial and solitary lifecycle phases, and some social parasite species, they are especially interesting models to understand social evolution, behavior, and ecology. Reports of many species in decline point to pathogen transmission, habitat loss, pesticide usage, and global climate change, as interconnected causes. These threats to bumblebee diversity make our reliance on a handful of well-studied species for agricultural pollination particularly precarious. To broadly sample bumblebee genomic and phenotypic diversity, we de novo sequenced and assembled the genomes of 17 species, representing all 15 subgenera, producing the first genus-wide quantification of genetic and genomic variation potentially underlying key ecological and behavioral traits. The species phylogeny resolves subgenera relationships while incomplete lineage sorting likely drives high levels of gene tree discordance. Five chromosome-level assemblies show a stable 18-chromosome karyotype, with major rearrangements creating 25 chromosomes in social parasites. Differential transposable element activity drives changes in genome sizes, with putative domestications of repetitive sequences influencing gene coding and regulatory potential. Dynamically evolving gene families and signatures of positive selection point to genus-wide variation in processes linked to foraging, diet and metabolism, immunity and detoxification, as well as adaptations for life at high altitudes. These high-quality genomic resources capture natural genetic and phenotypic variation across bumblebees, offering new opportunities to advance our understanding of their remarkable ecological success and to identify and manage current and future threats.
Date: 2020 May 31
arXiv: 2020.05.29.122879
34P. Best practices for genome-wide RNA structure analysis: combination of mutational profiles and drop-off information
Novoa, Beaudoin, Giraldez, Mattick, Kellis
Genome-wide RNA structure maps have recently become available through the coupling of in vivo chemical probing reagents with next-generation sequencing. Initial analyses relied on the identification of truncated reverse transcription reads to identify the chemically modified nucleotides, but recent studies have shown that mutational signatures can also be used. While these two methods have been employed interchangeably, here we show that they actually provide complementary information. Consequently, analyses using exclusively one of the two methodologies may disregard a significant portion of the structural information. We also show that the identity and sequence environment of the modified nucleotide greatly affect the odds of introducing a mismatch or causing reverse transcriptase drop-off. Finally, we identify specific mismatch signatures generated by dimethyl sulfate probing that can be exploited to remove false positives typically produced in RNA structurome analyses, and how these signatures vary depending on the reverse transcription enzyme used.
Date: 2020 Mar 31
bioRxiv: 176883.0
33P. High-Throughput 5' UTR Engineering for Enhanced Protein Production in Non-Viral Gene Therapies
Cao, Novoa, Zhang, Chen, Liu, Choi, Wong, Wehrspaun, Kellis, Lu
Despite significant clinical progress in cell and gene therapies, maximizing protein expression in order to enhance potency remains a major challenge. One approach to increase protein expression is by optimizing translation through the engineering of 5' untranslated regions (5' UTRs). Here, we developed a high-throughput strategy to design, screen, and optimize novel 5'UTRs that enhance protein expression from a strong human cytomegalovirus (CMV) promoter. We first identified naturally occurring 5' UTRs with high translation efficiencies and used this information with in silico genetic algorithms to generate synthetic 5' UTRs. A total of ∼12,000 5' UTRs were then screened using a recombinase-mediated integration strategy that greatly enhances the sensitivity of high-throughput screens by eliminating copy number and position effects that limit lentiviral approaches. Using this approach, we identified three synthetic 5' UTRs that outperformed commonly used non-viral gene therapy plasmids in expressing protein payloads. Furthermore, combinatorial assembly of these 5' UTRs enabled even higher protein expression than obtained with each individual 5' UTR. In summary, we demonstrate that high-throughput screening of 5' UTR libraries with recombinase-mediated integration can identify genetic elements that enhance protein expression, which should have numerous applications for engineered cell and gene therapies.
Date: 2020 Mar 25
arXiv: 2020.03.24.006486
32P. Thousands of novel unannotated proteins expand the MHC I immunopeptidome in cancer
Ouspenskaia, Law, Clauser, Klaeger, Sarkizova, Aguet, Li, Christian, Knisbacher, Le, Hartigan, Keshishian, Apffel, Oliveira, Zhang, Ting Chow, Ji, Jungreis, Shukla, Bachireddy, Kellis, Getz, Hacohen, Keskin, Carr, Wu, Regev
Tumor epitopes – peptides that are presented on surface-bound MHC I proteins - provide targets for cancer immunotherapy and have been identified extensively in the annotated protein-coding regions of the genome. Motivated by the recent discovery of translated novel unannotated open reading frames (nuORFs) using ribosome profiling (Ribo-seq), we hypothesized that cancer-associated processes could generate nuORFs that can serve as a new source of tumor antigens that harbor somatic mutations or show tumor-specific expression. To identify cancer-specific nuORFs, we generated Ribo-seq profiles for 29 malignant and healthy samples, developed a sensitive analytic approach for hierarchical ORF prediction, and constructed a high-confidence database of translated nuORFs across tissues. Peptides from 3,555 unique translated nuORFs were presented on MHC I, based on analysis of an extensive dataset of MHC I-bound peptides detected by mass spectrometry, with >20-fold more nuORF peptides detected in the MHC I immunopeptidomes compared to whole proteomes. We further detected somatic mutations in nuORFs of cancer samples and identified nuORFs with tumor-specific translation in melanoma, chronic lymphocytic leukemia and glioblastoma. NuORFs thus expand the pool of MHC I-presented, tumor-specific peptides, targetable by immunotherapies.
Date: 2020 Mar 5
arXiv: 2020.02.12.945840
31P. Correlating predicted epigenetic marks with expression data to find interactions between SNPs and genes
Despinasse, Park, Lapi, Kellis
Despite all the work done, mapping GWAS SNPs in non-coding regions to their target genes remains a challenge. The SNP can be associated with target genes by eQTL analysis. Here we introduce a method to make these eQTLs more robust. Instead of correlating the gene expression with the SNP value like in eQTLs, we correlate it with epigenomic data. This epigenomic data is very expensive and noisy. We therefore predict the epigenomic data from the DNA sequence using the deep learning framework DeepSEA (Zhou and Troyanskaya, 2015).
Date: 2020 Feb 29
arXiv: 2020.02.29.970962
30P. Integrative analysis of 10,000 epigenomic maps across 800 samples for regulatory genomics and disease dissection
Adsera, Park, Meuleman, Kellis
To help elucidate genetic variants underlying complex traits, we develop EpiMap, a compendium of 833 reference epigenomes across 18 uniformly-processed and computationally-completed assays. We define chromatin states, high-resolution enhancers, activity patterns, enhancer modules, upstream regulators, and downstream target gene functions. We annotate 30,247 genetic variants associated with 534 traits, recognize principal and partner tissues underlying each trait, infer trait-tissue, tissue-tissue and trait-trait relationships, and partition multifactorial traits into their tissue-specific contributing factors. Our results demonstrate the importance of dense, rich, and high-resolution epigenomic annotations for complex trait dissection, and yield numerous new insights for understanding the molecular basis of human disease.
Date: 2019 Oct 19
bioRxiv: 810291.0
29P. Plasma-derived exosomal analysis and deconvolution enables prediction and tracking of melanoma checkpoint blockade response
Shi, Kasumova, Michaud, Cintolo-Gonzales, Martínez, Ohmura, Mehta, Chien, Frederick, Cohen, Plana, Johnson, Flaherty, Sullivan, Kellis, Boland
Immune checkpoint inhibitors (ICI) have demonstrated promising therapeutic benefit although a majority will not respond. Here we identify and validate predictive biomarkers from plasma-derived exosomes that allow non-invasive monitoring of tumor intrinsic and host immune status and prediction of ICI success. Transcriptomic profiling of peripheral blood bulk exosomes and tumors from a discovery cohort of 50 patients with metastatic melanoma treated with ICI was undertaken; a further validation cohort of 30 patients was utilized to validate findings from the discovery cohort. We designed a Bayesian probabilistic model to partition bulk exosomes into tumor-specific and non-tumor-specific proportions. Exosomal RNA signatures exhibit significant correlations with tumor transcriptomes. Exosomal profiles reflect several key biological drivers of ICI resistance or melanoma progression, exhibit significantly differentially expressed genes and pathways, and correlate with and are predictive of clinical response to therapy. Our deconvolution model estimates contributions from tumor and non-tumor sources, enabling more precise interpretation of differentially-expressed genes and pathways. Exosomal RNA-seq mutational information can be used to segregate responders and non-responders. Peripheral blood-derived exosomes can serve as a non-invasive biomarker to jointly probe tumor-intrinsic and immune changes to ICI, and can potentially function as predictive markers of ICI responsiveness and a monitoring tool for tumor persistence and immune activation. We use transcriptomic analysis of bulk, non-selected, peripheral blood derived exosomes to reveal both tumor-intrinsic and immune-derived signatures predictive of early response to immune checkpoint inhibitor therapy. We develop a novel computational model to classify exosomal transcripts into tumor and non-tumor components and establish relevance in immune checkpoint blockade therapy. We show that tumor driver load from RNA-seq mutational calls are significantly different between responders and non-responders.
Date: 2018 Oct 18
bioRxiv: 809699.0
28P. A multiresolution framework to characterize single-cell state landscapes
Mohammadi, Davila-Velderrain, Kellis
Dissecting the cellular heterogeneity embedded in single-cell transcriptomic data is challenging. Although a large number of methods and approaches exist, robustly identifying underlying cell states and their associations is still a major challenge; given the nonexclusive and dynamic influence of multiple unknown sources of variability, the existence of state continuum at the time-scale of observation, and the inevitable snapshot nature of experiments. As a way to address some of these challenges, here we introduce ACTIONet, a comprehensive framework that combines archetypal analysis and network theory to provide a ready-to-use analytical approach for multiresolution single-cell state characterization. ACTIONet uses multilevel matrix decomposition and network reconstruction to simultaneously learn cell state patterns, quantify single-cell states, and reconstruct a reproducible structural representation of the transcriptional state space that is geometrically mapped to a color space. A color-enhanced quantitative view of cell states enables novel visualization, prediction, and annotation approaches. Using data from multiple tissues, organisms, and developmental conditions, we illustrate how ACTIONet facilitates the reconstruction and exploration of single-cell state landscapes.
Date: 2019 Sep 6
bioRxiv: 746339.0
27P. Analysis of Genetically Regulated Gene Expression identifies a trauma type specific PTSD gene, SNRNP35
Huckins, Breen, Chatzinakos, Hartmann, Klengel, daSilvaAlmeida, Dobbyn, Girdhar, Hoffman, Klengel, Logue, Lori, Morrison, Nguyen, Park, Ruderfer, Sloofman, vanRooij, PTSD_PGC, Baker, Chen, Cox, Duncan, Geyer, Glatt, Im, Maihofer, Risbrough, Smoller, Stein, Yehuda, Liberzon, Koenen, Jovanovic, Kellis, Miller, Bacanu, Nievergelt, Buxbaum, Sklar, Ressler, Stahl, Daskalakis
PTSD has significant genetic heritability; however, it is unclear how genetic risk influences tissue-specific gene expression. We used brain and non-brain transcriptomic imputation models to impute genetically regulated gene expression (GReX) in 9,087 PTSD-cases and 23,811 controls and identified thirteen significant GReX-PTSD associations. The results suggest substantial genetic heterogeneity between civilian and military PTSD cohorts. The top study-wide significant PTSD-association was with predicted downregulation of the Small Nuclear Ribonucleoprotein U11/U12 Subunit 35 (SNRNP35) in the BA9 region of the prefrontal cortex (PFC) in military cohorts. In peripheral leukocytes from 175 U.S. Marines, the observed PTSD differential gene expression correlated with the predicted blood GReX differences for these individuals, and deployment stress downregulated SNRNP35 expression, primarily in Marines with post-deployment PTSD. SNRNP35 is a subunit of the minor spliceosome complex and SNRNP35 knockdown in cells validated its functional importance in U12-intron splicing. Finally, mimicking acute activation of the endogenous stress axis in mice downregulated PFC Snrnp35 expression.
Date: 2019 Mar 19
bioRxiv: 581124.0
26P. A causal inference framework for estimating genetic variance and pleiotropy from GWAS summary data
Park, He, Kellis
Much of research in genome-wide association studies has only searched for significantly associated signals without explicitly removing unwanted source of variation. Confounder correction is a necessary step to reveal causal effects, but often skipped in a summary-based analysis. We present a novel causal inference algorithm that controls unwanted sources in genetic variance and covariance estimation tasks. We demonstrate substantially improved statistical power and accuracy in extensive simulations. In real-world applications on the UK biobank summary statistics data, our method recapitulates well-known pleiotropic modules, suggesting new insights into biobank-scale GWAS analysis.
Date: 2019 Jan 28
bioRxiv: 531673.0
25P. Causal Mediation Analysis Leveraging Multiple Types of Summary Statistics Data
Park, Sarkar, Nguyen, Kellis
Summary statistics of genome-wide association studies (GWAS) teach causal relationship between millions of genetic markers and tens and thousands of phenotypes. However, underlying biological mechanisms are yet to be elucidated. We can achieve necessary interpretation of GWAS in a causal mediation framework, looking to establish a sparse set of mediators between genetic and downstream variables, but there are several challenges. Unlike existing methods rely on strong and unrealistic assumptions, we tackle practical challenges within a principled summary-based causal inference framework. We analyzed the proposed methods in extensive simulations generated from real-world genetic data. We demonstrated only our approach can accurately redeem causal genes, even without knowing actual individual-level data, despite the presence of competing non-causal trails.
Date: 2019 Jan 24
arXiv: 1901.0854
24P. Simultaneous profiling of DNA methylation and chromatin architecture in mixed populations and in single cells
Li, Liu, Zhang, Fang, Kellis, Ren
Dynamic DNA methylation and three-dimensional chromatin architecture compose a major portion of the epigenome and play an essential role in tissue specific gene expression programs. Currently, DNA methylation and chromatin organization are generally profiled in separate assays. Here, we report Methyl-HiC, a method combining in situ Hi-C and whole genome bisulfite sequencing (WGBS) to simultaneously capture chromosome conformation and DNA methylome in a single assay. Methyl-HiC analysis of mouse embryonic stem cells reveals coordinated DNA methylation between distant yet spatially proximal genomic regions. Extension of Methyl-HiC to single cells further enables delineation of the heterogeneity of both chromosomal conformation and DNA methylation in a mixed cell population, and uncovers increased dynamics of chromatin contacts and decreased stochasticity in DNA methylation in genomic regions that replicate early during cell cycle.
Date: 2018 Nov 14
bioRxiv: 470963.0
23P. A latent topic model for mining heterogenous non-randomly missing electronic health records data
Li, Kellis
Electronic health records (EHR) are rich heterogeneous collection of patient health information, whose broad adoption provides great opportunities for systematic health data mining. However, heterogeneous EHR data types and biased ascertainment impose computational challenges. Here, we present mixEHR, an unsupervised generative model integrating collaborative filtering and latent topic models, which jointly models the discrete distributions of data observation bias and actual data using latent disease-topic distributions. We apply mixEHR on 12.8 million phenotypic observations from the MIMIC dataset, and use it to reveal latent disease topics, interpret EHR results, impute missing data, and predict mortality in intensive care units. Using both simulation and real data, we show that mixEHR outperforms previous methods and reveals meaningful multi-disease insights.
Date: 2018 Nov 1
arXiv: 1811.00464
22P. Genie: A Secure, Transparent Sharing and Services Platform for Genetic and Health Data
Zhang, Kim, Liu, Nuckchadyy, Huangy, Masurkary, Zhang, Karnatiz, Martinezx, Hardjono, Kellis, Zhang
Artificial Intelligence (AI) incorporating genetic and medical information have been applied in disease risk prediction, unveiling disease mechanism, and advancing therapeutics. However, AI training relies on highly sensitive and private data which significantly limit their applications and robustness evaluation. Moreover, the data access management after sharing across organization heavily relies on legal restriction, and there is no guarantee in preventing data leaking after sharing. Here, we present Genie, a secure AI platform which allows AI models to be trained on medical data securely. The platform combines the security of Intel Software Guarded eXtensions (SGX), transparency of blockchain technology, and verifiability of open algorithms and source codes. Genie shares insights of genetic and medical data without exposing anyone's raw data. All data is instantly encrypted upon upload and contributed to the models that the user chooses. The usage of the model and the value generated from the genetic and health data will be tracked via a blockchain, giving the data transparent and immutable ownership.
Date: 2018 Nov 4
arXiv: 1811.01431
21P. Evidence for secondary-variant genetic burden and non-random distribution across biological modules in a recessive ciliopathy
Kousi, Soylemez, Ozanturk, Akle, Jungreis, Muller, Cassa, Brand, Rosenfeld, Wolf, Sadeghpour, McFadden, Lewis, Talkowski, Dollfus, Kellis, Davis, Sunyaev, Katsanis
The influence of genetic background on driver mutations is well established; however, the mechanisms by which the background interacts with Mendelian loci remains unclear. We performed a systematic secondary-variant burden analysis of two independent Bardet-Biedl syndrome (BBS) cohorts with known recessive biallelic pathogenic mutations in one of 17 BBS genes for each individual. We observed a significant enrichment of trans-acting rare nonsynonymous secondary variants compared to either population controls or to a cohort of individuals with a non-BBS diagnosis and recessive variants in the same gene set. Strikingly, we found a significant over-representation of secondary alleles in chaperonin-encoding genes, a finding corroborated by the observation of epistatic interactions involving this complex in vivo. These data indicate a complex genetic architecture for BBS that informs the biological properties of disease modules and presents a model paradigm for secondary-variant burden analysis in recessive disorders.
Date: 2018 Jul 5
bioRxiv: 362707.0
20P. A gene expression atlas of embryonic neurogenesis in Drosophila reveals complex spatiotemporal regulation of lncRNAs
McCorkindale, Wahle, Werner, Jungreis, Menzel, Shukla, Abreu, Irizarry, Meyer, Kellis, Zinzen
Cell type specification during early nervous system development in Drosophila melanogaster requires precise regulation of gene expression in time and space. Resolving the programs driving neurogenesis has been a major challenge owing to the complexity and rapidity with which distinct cell populations arise. To resolve the cell type-specific gene expression dynamics in early nervous system development, we have sequenced the transcriptomes of purified neurogenic cell types across consecutive time points covering critical events in neurogenesis. The resulting gene expression atlas comprises a detailed resource of global transcriptome dynamics that permits systematic analysis of how cells in the nervous system acquire distinct fates. We resolve known gene expression dynamics and uncover novel expression signatures for hundreds of genes among diverse neurogenic cell types, most of which remain unstudied. We also identified a set of conserved and processed long-noncoding RNAs (lncRNAs) that exhibit spatiotemporal expression during neurogenesis with exquisite specificity. LncRNA expression is highly dynamic and demarcates specific subpopulations within neurogenic cell types. Our spatiotemporal transcriptome atlas provides a comprehensive resource to investigate the function of coding genes and noncoding RNAs during critical stages of early neurogenesis.
Date: 2018 Dec 27
bioRxiv: 483461.0
19P. DECODE-ing sparsity patterns in single-cell RNA-seq
Mohammadi, Davila-Velderrain, Kellis, Gram
An inherent challenge in interpreting single-cell transcriptomic data is the high frequency of zero values. This phenomenon has been attributed to both biological and technical sources, although the extent of the contribution of each remains unclear. Here, we show that the underlying gene presence/absence sparsity patterns are by themselves highly informative. We develop an algorithm, called DECODE, to assess the extent of joint presence/absence of genes across different cells, and to infer a gene dependency network. We show that this network captures biologically-meaningful pathways, cell-type specific modules, and connectivity patterns characteristic of complex networks. We develop a model that uses this network to discriminate biological vs. technical zeros, by exploiting each gene's local network neighborhood. For inferred non-biological zeros, we build a predictive model that imputes the missing value of each gene based on activity patterns of its most informative neighbors. We show that our framework accurately infers gene-gene functional dependencies, pinpoints technical zeros, and predicts biologically-meaningful missing values in three diverse datasets.
Date: 2018 Mar 9
bioRxiv: 241646.0
18P. Predicting trait regulators by identifying co-localization of DNA binding and GWAS variants in regulatory regions
Quon, Feizi, Marbach, Claussnitzer, Kellis
Genomic regions associated with complex traits and diseases are primarily located in non-coding regions of the genome and have unknown mechanism of action. A critical step to understanding the genetics of complex traits is to fine-map each associated locus; that is, to find the causal variant(s) that underlie genetic associations with a trait. Fine-mapping approaches are currently focused on identifying genomic annotations, such as transcription factor binding sites, which are enriched in direct overlap with candidate causal variants. We introduce CONVERGE, the first computational tool to search for co-localization of GWAS causal variants with transcription factor binding sites in the same regulatory regions, without requiring direct overlap. As a proof of principle, we demonstrate that CONVERGE is able to identify five novel regulators of type 2 diabetes which subsequently validated in knockdown experiments in pancreatic beta cells, while existing fine-mapping methods were unable to find any statistically significant regulators. CONVERGE also recovers more established regulators for total cholesterol compared to other fine-mapping methods. CONVERGE is therefore unique and complementary to existing fine-mapping methods and is useful for exploring the regulatory architecture of complex traits.
Date: 2018 Nov 11
bioRxiv: 467852.0
17P. Modeling prediction error improves power of transcriptome-wide association studies
Bhutani, Sarkar, Park, Kellis, Schork
Transcriptome-wide association studies (TWAS) test for associations between imputed gene expression levels and phenotypes in GWAS cohorts using models of transcriptional regulation learned from reference transcriptomes. However, current methods for TWAS only use point estimates of imputed expression and ignore uncertainty in the prediction. We develop a novel two-stage Bayesian regression method which incorporates uncertainty in imputed gene expression and achieves higher power to detect TWAS genes than existing TWAS methods as well as standard methods based on missing value and measurement error theory. We apply our method to GTEx whole blood transcriptomes and GWAS cohorts for seven diseases from the Wellcome Trust Case Control Consortium and find 45 TWAS genes, of which 17 do not overlap previously reported case-control GWAS or differential expression associations. Surprisingly, we replicate only 2 of 40 previously reported TWAS genes after accounting for uncertainty in the prediction.
Date: 2017 Feb 14
bioRxiv: 108316.0
16P. Multi-tissue polygenic models for transcriptome-wide association studies
Park, Sarkar, Bhutani, Kellis
Transcriptome-wide association studies (TWAS) have proven to be a powerful tool to identify genes associated with human diseases by aggregating cis-regulatory effects on gene expression. However, TWAS relies on building predictive models of gene expression, which are sensitive to the sample size and tissue on which they are trained. The Gene Tissue Expression Project has produced reference transcriptomes across 53 human tissues and cell types; however, the data is highly sparse, making it difficult to build polygenic models in relevant tissues for TWAS. Here, we propose ‘fQTL', a multi-tissue, multivariate model for mapping expression quantitative trait loci and predicting gene expression. Our model decomposes eQTL effects into SNP-specific and tissue-specific components, pooling information across relevant tissues to effectively boost sample sizes. In simulation, we demonstrate that our multi-tissue approach outperforms single-tissue approaches in identifying causal eQTLs and tissues of action. Using our method, we fit polygenic models for 13,461 genes, characterized the tissue-specificity of the learned cis-eQTLs, and performed TWAS for Alzheimer's disease and schizophrenia, identifying 107 and 382 associated genes, respectively.
Date: 2017 Feb 10
bioRxiv: 107623.0
15P. Discovery and characterization of coding and non-coding driver mutations in more than 2,500 whole cancer genomes
Rheinbay, Nielsen, Abascal, Tiao, Hornshoj, Hess, Pedersen, Feuerbach, Sabarinathan, Madsen, KIM, Mularoni, Shuai, Camaioni, Herrmann, Maruvka, Shen, Amin, Bertl, Dhingra, Diamanti, Gonzalez-Perez, Guo, Haradhvala, Isaev, Juul, Komorowski, kumar, Lee, Lochovsky, Liu, Pich, Tamborero, Umer, Uusküla-Reimand, Wadelius, Wadi, Zhang, Boroevich, Carlevaro-Fita, Chakravarty, Chan, Fonseca, Hamilton, Hong, Kahles, Kim, Lehmann, Johnson, Kahraman, Park, Saksena, Sieverling, Sinnott-Armstrong, Campbell, Hobolth, Kellis, Lawrence, Raphael, Rubin, Sander, Stein, Stuart, Tsunoda, Wheeler, Johnson, Reimand, Gerstein, Khurana, Lopez-Bigas, Martincorena, Pedersen, Getz, PCAWG Drivers and Functional Interpretation Group, ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Net
Discovery of cancer drivers has traditionally focused on the identification of protein-coding genes. Here we present a comprehensive analysis of putative cancer driver mutations in both protein-coding and non-coding genomic regions across >2,500 whole cancer genomes from the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium. We developed a statistically rigorous strategy for combining significance levels from multiple driver discovery methods and demonstrate that the integrated results overcome limitations of individual methods. We combined this strategy with careful filtering and applied it to protein-coding genes, promoters, untranslated regions (UTRs), distal enhancers and non-coding RNAs. These analyses redefine the landscape of non-coding driver mutations in cancer genomes, confirming a few previously reported elements and raising doubts about others, while identifying novel candidate elements across 27 cancer types. Novel recurrent events were found in the promoters or 5'UTRs of TP53, RFTN1, RNF34, and MTG2, in the 3'UTRs of NFKBIZ and TOB1, and in the non-coding RNA RMRP. We provide evidence that the previously reported non-coding RNAs NEAT1 and MALAT1 may be subject to a localized mutational process. Perhaps the most striking finding is the relative paucity of point mutations driving cancer in non-coding genes and regulatory elements. Though we have limited power to discover infrequent non-coding drivers in individual cohorts, combined analysis of promoters of known cancer genes show little excess of mutations beyond TERT.
Date: 2017 Dec 23
bioRxiv: 237313.0
14P. Integrative construction of regulatory region networks in 127 human reference epigenomes by matrix factorization
Liu, Davila-Velderrain, Zhang, Kellis
Despite large experimental and computational efforts aiming to dissect the mechanisms underlying disease risk, mapping cis-regulatory elements to target genes remains a challenge. Here, we introduce a matrix factorization framework to integrate physical and functional interaction data of genomic segments. The framework was used to predict a regulatory network of chromatin interaction edges linking more than 20,000 promoters and 1.8 million enhancers across 127 human reference epigenomes, including edges that are present in any of the input datasets. Our network integrates functional evidence of correlated activity patterns from epigenomic data and physical evidence of chromatin interactions. An important contribution of this work is the representation of heterogeneous data with different qualities as networks. We show that the unbiased integration of independent data sources suggestive of regulatory interactions produces meaningful associations supported by existing functional and physical evidence, correlating with expected independent biological features.
Date: 2017 Nov 10
bioRxiv: 217588.0
13P. A probabilistic framework to dissect functional cell-type-specific regulatory elements and risk loci underlying the genetics of complex traits
Li, Davila-Velderrain, Kellis
Dissecting the physiological circuitry underlying diverse human complex traits associated with heritable common mutations is an ongoing effort. The primary challenge involves identifying the relevant cell types and the causal variants among the vast majority of the associated mutations in the noncoding regions. To address this challenge, we developed an efficient probabilistic framework. First, we propose a sparse group-guided learning algorithm to infer cell-type-specific enrichments. Second, we propose a fine-mapping Bayesian model that incorporates as Bayesian priors the sparse enrichments to infer risk variants. Using the proposed framework to analyze 32 complex human traits revealed meaningful tissue-specific epigenomic enrichments indicative of the relevant disease pathologies. The prioritized variants exhibit prominent tissue-specific epigenomic signatures and significant enrichments for eQTL and conserved elements. Together, we demonstrate the general benefits of the proposed integrative framework in elucidating meaningful tissue-specific epigenomic elements from large-scale correlated annotations and the implicated functional variants for future experimental interrogation.
Date: 2017 Oct 24
bioRxiv: 59345.0
12P. Genome-wide regulatory model from MPRA data predicts functional regions, eQTLs, and GWAS hits
Li, Shi, Tewhey, Sabeti, Ernst, Kellis
Massively-parallel reporter assays (MPRA) enable unprecedented opportunities to test for regulatory activity of thousands of regulatory sequences. However, MPRA only assay a subset of the genome thus limiting their applicability for genome-wide functional annotations. To overcome this limitation, we have used existing MPRA datasets to train a machine learning model that uses DNA sequence information, regulatory motif annotations, evolutionary conservation, and epigenomic information to predict genomic regions that show enhancer activity when tested in MPRA assays. We used the resulting model to generate global predictions of regulatory activity at single-nucleotide resolution across 14 million common variants. We find that genetic variants with stronger predicted regulatory activity show significantly lower minor allele frequency, indicative of evolutionary selection within the human population. They also show higher overlap with eQTL annotations across multiple tissues relative to the background SNPs, indicating that their perturbations in vivo more frequently result in changes in gene expression. In addition, they are more frequently associated with trait-associated SNPs from genome-wide association studies (GWAS), enabling us to prioritize genetic variants that are more likely to be causal based on their predicted regulatory activity. Lastly, we use our model to compare MPRA inferences across cell types and platforms and to prioritize the assays most predictive of MPRA assay results, including cell-dependent DNase hypersensitivity sites and transcription factors known to be active in the tested cell types. Our results indicate that high-throughput testing of thousands of putative regions, coupled with regulatory predictions across millions of sites, presents a powerful strategy for systematic annotation of genomic regions and genetic variants.
Date: 2017 Feb 20
bioRxiv: 110171.0
11P. Best practices for genome-wide RNA structure analysis: combination of mutational profiles and drop-off information
Novoa, Beaudoin, Giraldez, Mattick, Kellis
Genome-wide RNA structure maps have recently become available through the coupling of in vivo chemical probing reagents with next-generation sequencing. Initial analyses relied on the identification of truncated reverse transcription reads to identify the chemically modified nucleotides, but recent studies have shown that mutational signatures can also be used. While these two methods have been employed interchangeably, here we show that they actually provide complementary information. Consequently, analyses using exclusively one of the two methodologies may disregard a significant portion of the structural information. We find that the identity and sequence environment of the modified nucleotide greatly affects the odds of introducing a mismatch or causing reverse transcriptase drop-off. Finally, we identify specific mismatch signatures generated by dimethyl sulfate probing that can be used to remove false positives typically produced in RNA structurome analyses, and how these signatures vary depending on the reverse transcription enzyme used.
Date: 2017 Aug 21
bioRxiv: 176883.0
10P. A Bayesian approach to mediation analysis predicts 206 causal target genes in Alzheimer's disease
Park, Sarkar, He, Davila-Velderrain, De Jager, Kellis
Characterizing the intermediate phenotypes, such as gene expression, that mediate genetic effects on complex diseases is a fundamental problem in human genetics. Existing methods utilize genotypic data and summary statistics to identify putative disease genes, but cannot distinguish pleiotropy from causal mediation and are limited by overly strong assumptions about the data. To overcome these limitations, we develop Causal Multivariate Mediation within Extended Linkage disequilibrium (CaMMEL), a novel Bayesian inference framework to jointly model multiple mediated and unmediated effects relying only on summary statistics. We show in simulation that CaMMEL accurately distinguishes between mediating and pleiotropic genes unlike existing methods. We applied CaMMEL to Alzheimer's disease (AD) and found 206 causal genes in sub-threshold loci (p < 1e-4). We prioritized 21 genes which mediate at least 5% of local genetic variance, disrupting innate immune pathways in AD.
Date: 2017 Dec 1
bioRxiv: 219428.0
9P. Network Infusion to Infer Information Sources in Networks
Feizi, Medard, Quon, Kellis, Duffy
Several significant models have been developed that enable the study of diffusion of signals across biological, social and engineered networks. Within these established frameworks, the inverse problem of identifying the source of the propagated signal is challenging, owing to the numerous alternative possibilities for signal progression through the network. In real world networks, the challenge of determining sources is compounded as the true propagation dynamics are typically unknown, and when they have been directly measured, they rarely conform to the assumptions of any of the well-studied models. In this paper we introduce a method called Network Infusion (NI) that has been designed to circumvent these issues, making source inference practical for large, complex real world networks. The key idea is that to infer the source node in the network, full characterization of diffusion dynamics, in many cases, may not be necessary. This objective is achieved by creating a diffusion kernel that well-approximates standard diffusion models, but lends itself to inversion, by design, via likelihood maximization or error minimization. We apply NI for both single-source and multi-source diffusion, for both single-snapshot and multi-snapshot observations, and for both homogeneous and heterogeneous diffusion setups. We prove the mean-field optimality of NI for different scenarios, and demonstrate its effectiveness over several synthetic networks. Moreover, we apply NI to a real-data application, identifying news sources in the Digg social network, and demonstrate the effectiveness of NI compared to existing methods. Finally, we propose an integrative source inference framework that combines NI with a distance centrality-based method, which leads to a robust performance in cases where the underlying dynamics are unknown.
Date: 2016 Jun 23
arXiv: 1606.07383
8P. Functional enrichments of disease variants across thousands of independent loci in eight diseases
Sarkar, Ward, Kellis
For most complex traits, known genetic associations only explain a small fraction of the narrow sense heritability prompting intense debate on the genetic basis of complex traits. Joint analysis of all common variants together explains much of this missing heritability and reveals that large numbers of weakly associated loci are enriched in regulatory regions, but fails to identify specific regions or biological pathways. Here, we use epigenomic annotations across 127 tissues and cell types to investigate weak regulatory associations, the specific enhancers they reside in, their downstream target genes, their upstream regulators, and the biological pathways they disrupt in eight common diseases. We show weak associations are significantly enriched in disease-relevant regulatory regions across thousands of independent loci. We develop methods to control for LD between weak associations and overlap between annotations. We show that weak non-coding associations are additionally enriched in relevant biological pathways implicating additional downstream target genes and upstream disease-specific master regulators. Our results can help guide the discovery of biologically meaningful, but currently undetectable regulatory loci underlying a number of common diseases.
Date: 2016 Apr 11
bioRxiv: 48066.0
7P. Convergence of dispersed regulatory mutations predicts driver genes in prostate cancer
Sallari, Sinnott-Armstrong, French, Kron, Ho, Moore, Stambolic, Edwards, Lupien, Kellis
Cancer sequencing predicts driver genes using recurrent protein-altering mutations, but detecting recurrence for non-coding mutations remains unsolved. Here, we present a convergence framework for recurrence analysis of non-coding mutations, using three-dimensional co-localization of epigenomically-defined regions. We define the regulatory plexus of each gene as its cell-type-specific three-dimensional gene-regulatory neighborhood, inferred using Hi-C chromosomal interactions and chromatin state annotations. Using 16 matched tumor-normal prostate transcriptomes, we predict tumor-upregulated genes, and find enriched plexus mutations in distal regulatory regions normally repressed in prostate, suggesting out-of-context de-repression. Using 55 matched tumor-normal prostate genomes, we predict 15 driver genes by convergence of dispersed, low-frequency mutations into high-frequency dysregulatory events along prostate-specific plexi, controlling for mutational heterogeneity across regions, chromatin states, and patients. These play roles in growth signaling, immune evasion, mitochondrial function, and vascularization, suggesting higher-order pathway-level convergence. We experimentally validate the PLCB4 plexus and its ability to affect the canonical PI3K cancer pathway.
Date: 2016 Dec 30
bioRxiv: 97451.0
6P. Network Maximal Correlation
Feizi, Makhdoumi, Duffy, Medard, Kellis
We introduce Network Maximal Correlation (NMC) as a multivariate measure of nonlinear association among random variables. NMC is defined via an optimization that infers transformations of variables by maximizing aggregate inner products between transformed variables. For finite discrete and jointly Gaussian random variables, we characterize a solution of the NMC optimization using basis expansion of functions over appropriate basis functions. For finite discrete variables, we propose an algorithm based on alternating conditional expectation to determine NMC. Moreover we propose a distributed algorithm to compute an approximation of NMC for large and dense graphs using graph partitioning. For finite discrete variables, we show that the probability of discrepancy greater than any given level between NMC and NMC computed using empirical distributions decays exponentially fast as the sample size grows. For jointly Gaussian variables, we show that under some conditions the NMC optimization is an instance of the Max-Cut problem. We then illustrate an application of NMC in inference of graphical model for bijective functions of jointly Gaussian variables. Finally, we show NMC's utility in a data application of learning nonlinear dependencies among genes in a cancer dataset.
Date: 2016 Jun 15
arXiv: 1606.04789
5P. RiVIERA-beta: Joint Bayesian inference of risk variants and tissue-specific epigenomic enrichments across multiple complex human diseases
Li, Kellis
Genome wide association studies (GWAS) provide a powerful approach for uncovering disease-associated variants in human, but fine-mapping the causal variants remains a challenge. This is partly remedied by prioritization of disease-associated variants that overlap GWAS-enriched epigenomic annotations. Here, we introduce a new Bayesian model RiVIERA-beta (Risk Variant Inference using Epigenomic Reference Annotations) for inference of driver variants by modelling summary statistics p-values in Beta density function across multiple traits using hundreds of epigenomic annotations. In simulation, RiVIERA-beta promising power in detecting causal variants and causal annotations, the multi-trait joint inference further improved the detection power.} We applied RiVIERA-beta to model the existing GWAS summary statistics of 9 autoimmune diseases and Schizophrenia by jointly harnessing the potential causal enrichments among 848 tissue-specific epigenomics annotations from ENCODE/Roadmap consortium covering 127 cell/tissue types and 8 major epigenomic marks. RiVIERA-beta identified meaningful tissue-specific enrichments for enhancer regions defined by H3K4me1 and H3K27ac for Blood T-Cell specifically in the 9 autoimmune diseases and Brain-specific enhancer activities exclusively in Schizophrenia. Moreover, the variants from the 95% credible sets exhibited high conservation and enrichments for GTEx whole-blood eQTLs located within transcription-factor-binding-sites and DNA-hypersensitive-sites. Furthermore, joint modeling the nine immune traits by simultaneously inferring and exploiting the underlying epigenomic correlation between traits further improved the functional enrichments compared to single-trait models.
Date: 2016 Jul 16
bioRxiv: 59329.0
4P. Various localized epigenetic marks predict expression across 54 samples and reveal underlying chromatin state enrichments
Devadas, Yen, Kellis
Here, we predict gene expression from epigenetic features based on public data available through the Epigenome Roadmap Project. This rich new dataset includes samples from primary tissues, which to our knowledge have not previously been studied in this context. Specifically, we used computational machine learning algorithms on five histone modifications to predict gene expression in a variety of samples. Our models reveal a high predictive accuracy, especially in cell cultures, with predictive ability dependent on sample type and anatomy. The relative importance of each histone mark feature varied across samples. We localized each histone mark signal to its relevant region, revealing that chromatin state enrichment varies greatly between histone marks. Our results provide several novel insights into epigenetic regulation of transcription in new contexts.
Date: 2015 Nov 3
bioRxiv: 30478.0
3P. A Perspective on Future Research Directions in Information Theory
Andrews, Dimakis, Dolecek, Effros, Medard, Milenkovic, Montanari, Vishwanath, Yeh, Berry, Duffy, Feizi, Kato, Kellis, Licht, Sorenson, Varshney, Vikalo
Information theory is rapidly approaching its 70th birthday. What are promising future directions for research in information theory? Where will information theory be having the most impact in 10-20 years? What new and emerging areas are ripe for the most impact, of the sort that information theory has had on the telecommunications industry over the last 60 years? How should the IEEE Information Theory Society promote high-risk new research directions and broaden the reach of information theory, while continuing to be true to its ideals and insisting on the intellectual rigor that makes its breakthroughs so powerful? These are some of the questions that an ad hoc committee (composed of the present authors) explored over the past two years. We have discussed and debated these questions, and solicited detailed inputs from experts in fields including genomics, biology, economics, and neuroscience. This report is the result of these discussions.
Date: 2015 Jul 20
arXiv: 1507.05941
2P. Phylogenetic Identification and Functional Characterization of Orthologs and Paralogs across Human, Mouse, Fly, and Worm
Wu, Bansal, Rasmussen, Herrero, Kellis
Model organisms can serve the biological and medical community by enabling the study of conserved gene families and pathways in experimentally-tractable systems. Their use, however, hinges on the ability to reliably identify evolutionary orthologs and paralogs with high accuracy, which can be a great challenge at both small and large evolutionary distances. Here, we present a phylogenomics-based approach for the identification of orthologous and paralogous genes in human, mouse, fly, and worm, which forms the foundation of the comparative analyses of the modENCODE and mouse ENCODE projects. We study a median of 16,101 genes across 2 mammalian genomes (human, mouse), 12 Drosophila genomes, 5 Caenorhabditis genomes, and an outgroup yeast genome, and demonstrate that accurate inference of evolutionary relationships and events across these species must account for frequent gene-tree topology errors due to both incomplete lineage sorting and insufficient phylogenetic signal. Furthermore, we show that integration of two separate phylogenomic pipelines yields increased accuracy, suggesting that their sources of error are independent, and finally, we leverage the resulting annotation of homologous genes to study the functional impact of gene duplication and loss in the context of rich gene expression and functional genomic datasets of the modENCODE, mouse ENCODE, and human ENCODE projects.
Date: 2014 May 31
bioRxiv: 5736.0
1P. Diversifying Sparsity Using Variational Determinantal Point Processes
Batmanghelich, Quon, Kulesza, Kellis, Golland, Bornn
We propose a novel diverse feature selection method based on determinantal point pro- cesses (DPPs). Our model enables one to flexibly define diversity based on the covari- ance of features (similar to orthogonal match- ing pursuit) or alternatively based on side information. We introduce our approach in the context of Bayesian sparse regression, employing a DPP as a variational approx- imation to the true spike and slab poste- rior distribution. We subsequently show how this variational DPP approximation general- izes and extends mean-field approximation, and can be learned efficiently by exploiting the fast sampling properties of DPPs. Our motivating application comes from bioinfor- matics, where we aim to identify a diverse set of genes whose expression profiles predict a tumor type where the diversity is defined with respect to a gene-gene interaction net- work. We also explore an application in spa- tial statistics. In both cases, we demonstrate that the proposed method yields significantly more diverse feature sets than classic sparse methods, without compromising accuracy.
Date: 2014 Nov 23
arXiv: 1411.6307