MIT Computational Biology Group - Our research in the news
Recent News Stories:
MIT News (not in Google News):
 |
Study: Movement disorder ALS and cognitive disorder FTLD show strong molecular overlaps
|
 |
Alzheimer's risk gene undermines insulation of brain's "wiring"
|
 |
Enabling AI-driven health advances without sacrificing patient privacy
|
 |
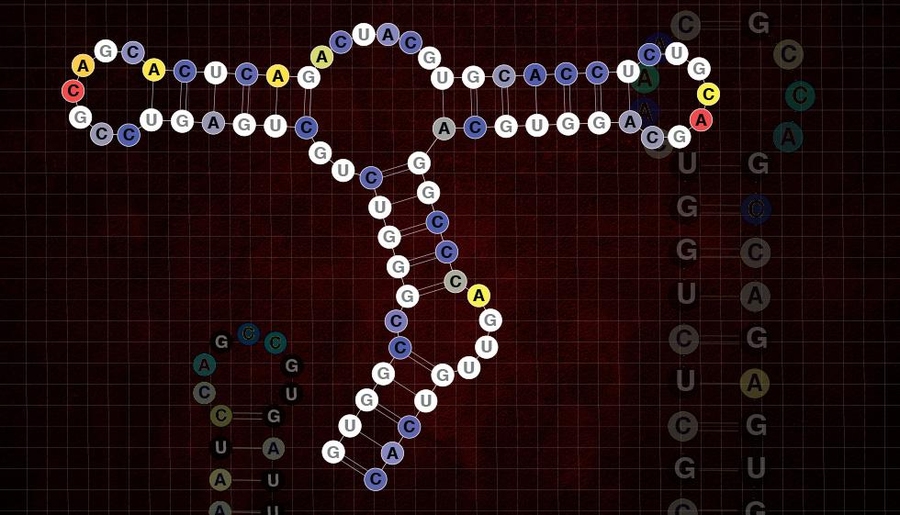
A comprehensive map of the SARS-CoV-2 genome
|
 |
Epigenomic map reveals circuitry of 30,000 human disease regions
|
 |
Study finds path for addressing Alzheimer's blood-brain barrier impairment
|
 |
Research highlights immune molecule's complex role in Huntington's disease
|
 |
Researching from home: Science stays social, even at a distance
|
 |
School of Engineering second quarter 2019 awards
|
 |
School of Engineering first quarter 2019 awards
|
 |
Alana gift to MIT launches Down syndrome research center, technology program for disabilities
|
 |
Daniel Zuo: Creative approaches to connectivity
|
 |
Four MIT students named 2017 Marshall Scholars
|
 |
Scene at MIT: Independence Day spectacular
|
 |
New approach to genetic analysis yields markers linked to complex diseases
|
 |
Creating postdoc connections
|
 |
MIT, White House co-sponsor workshop on big-data privacy
|
 |
Unusual suspects
|
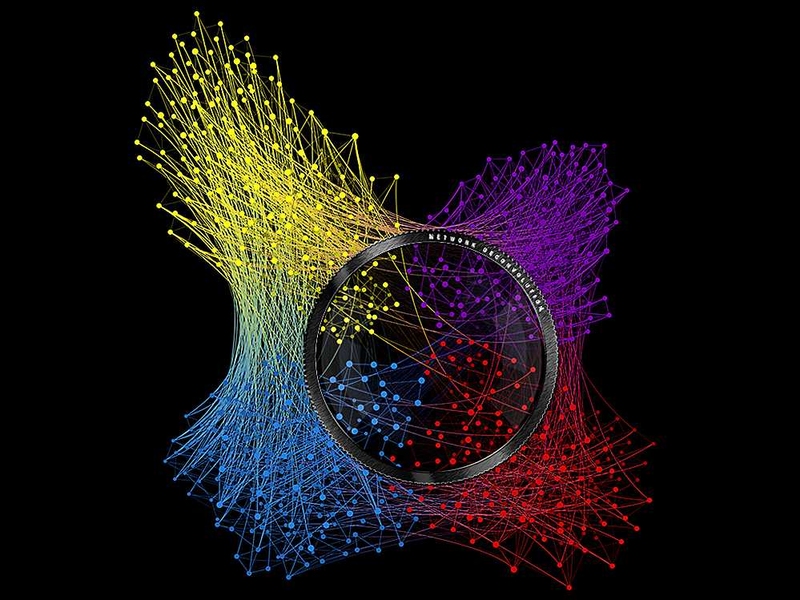 |
Researchers develop new method for understanding network connections
|
 |
Department snapshot: Electrical Engineering and Computer Science
|
 |
Researchers identify biochemical functions for most of the human genome
|
|
The wisdom of crowds
|
 |
Getting to the root of genetics
|
 |
Analysis of 29 mammals reveals genomic "dark matter'
|
 |
Meet the 2011 Kambourides Fellow
|
 |
7 win presidential early career honors
|
|
Study takes aim at opportunistic fungal pathogens
|
 |
Tales from the crib: MIT assistant publishes 20th children's book
|
|
Seven junior faculty named Sloan Research Fellows
|
 |
On the front lines of the genomic revolution
|
|
MIT reports new twist in microRNA biology
|
 |
Team analyzes genomes of 12 fly species
|
|
MIT faculty, alumni cited as top young innovators in Technology Review
|
|
EECS appoints 10 to professorships
|
 |
Next generation of researchers honored by Museum of Science
|
 |
Small fish yields big insights
|
 |
Yeast link to ancestral super-organism found
|
 |
Scientists find yeast link to ancestral super-organism
|
 |
Daffodils net thousands to fight cancer
|
Tweets by Manolis
Tweets by manoliskellisOlder News Stories:
- April 2013: Jason's paper in Genome Research
- March 2013: Pouya's paper in Genome Research with Jason Ernst and Tarjei Mikkelsen
- March 2013: Nisha's paper in PLoS CB
- Feb 2013: Loyal's paper in PNAS
- Dec 2012: Michael and Jason's paper on Chromatin States in the ENCODE project NAR
- Sept 2012: Luke's human selection paper appears in Science
- Covered in a Science and Technology story in The Economist
- Sept 2012: ENCODE project papers come out:
- Integrative paper in Nature
- Motif variation paper in Genome Biology
- GENCODE paper in Genome Research
- ChIP-Seq guidelines paper in Genome Research
- Mike's paper on Overlapping constraint elements within human protein-coding exons
- Irwin's paper on Stop codon Readhtrough in fly, human, and other animal genomes
- Jakob's paper on Structural RNA families in the human genome
- Read the MIT News and CSAIL story
- Paper 1: Chromatin Dynamics in human,
- Paper 2: Chromatin Landscape in Drosophila,
- Paper 3: Cis-regulatory map in Drosophila
- News story: Epigenomic findings illuminate veiled variants Haley Bridger, Broad Institute
- News story: Computational Biology Group Offers Look at Human & Fly Genome Regulation CSAIL news office
Videos
HHMI interview (2002)Matt's thesis defense (2010)
CRG symposium (2010)
Google News Archive
- Click on the above link to search a timeline of news stories for our group
- Note: Because some news stories move and links eventually fail, exceprts of the stories are linked with an asterisk below.
Epigenomics
12 Drosophila genomes - Fly comparative genomics
General Interviews
microRNAs
Regulatory Motifs - Human / Yeast
Yeast Whole-Genome Duplication
YeastReannotation
Old News (back when i was Kamvysselis)
Technology Review Top Young Innovators http://www.technologyreview.com/tr35/Profile.aspx?Cand=T&TRID=467 Since 1999, the editors of Technology Review have honored the young innovators whose inventions and research we find most exciting; today that collection is the TR35, a list of technologists and scientists, all under the age of 35. Their work--spanning medicine, computing, communications, electronics, nanotechnology, and more--is changing our world.[...] Manolis Kellis develops algorithms and techniques for analyzing the entire genomes of different species, the better to understand those genomes. Kellis began his PhD work with little knowledge of biology: his undergraduate degree is in computer science. For his thesis, he compared the genomes of four yeast species to identify all the genes and regulatory sequences in one of them--a project hes glad no one told him was believed to be impossible. Comparing the genomes of multiple closely related species has proved to be a powerful new tool for finding genes and the sequences that regulate them, and for learning about how genomes evolve (see "Finding Evolution's Signatures"). After validating his methods in yeast, Kellis has moved to the human genome, which he has so far compared with those of the mouse, rat, and dog. His work is providing an intimate understanding of the human genome that may give drug developers new points of entry in their attempts to combat viruses and other causes of disease. Museum of Science Award http://web.mit.edu/newsoffice/2004/museum-1117.html Though far from finished with their own work, three senior MIT researchers passed the torch to a new generation of scientists on Tuesday, Nov. 9 at the Museum of Science in Boston. For the past two years, the museum has named several young New England scientists as the "Next Generation" of revolutionary researchers whose work already has made a significant contribution to their field. This year, the three honorees all work in biotechnology at MIT. [...] Professor Eric Lander, founding director of the Eli and Edythe L. Broad Institute, whose mission is to create tools for genomic medicine, make them broadly available and use them to propel the understanding and treatment of disease, called it "a good and new experience for me to be part of the old generation." In his introduction of Kellis, Lander said he was impressed with the young scientist's enthusiasm and insights, referring to Kellis as an "extraordinary ball of energy." Kellis proved Lander's point by speaking enthusiastically about his work. He called it "using evolution to inform genomics, and using genomics to understand evolution." Kellis, who earned his Ph.D. in computer science from MIT, has developed new computational paradigms to help decipher DNA signals, understand gene regulation and clarify the evolutionary mechanisms of genomes. He has applied these tools to the yeast and human genomes, to systematically study all genes and regulatory elements. His work also showed that the yeast genome arose by whole-genome duplication, and that a similar event shaped the early vertabrate evolution of several fish. Kellis' "never-ending smile and unabashed optimism has always impressed me," Lander said. "This is definitely our future." Combining computer science with biology http://www.mitadmissions.org/topics/pulse/faculty_at_mit/prof_manolis_kellis_combining_1.shtml University professors are often portrayed as self-absorbed individuals that are too busy plotting their next breakthrough to pay any attention to their undergraduate students. While I have never encountered such a professor at MIT, Manolis Kellis, Assistant Professor of Computer Science, definitely destroys this all-too-prevalent stereotype. Last week, Prof. Kellis was honored as one of Technology Review's Top 35 Innovators Under 35 for his pioneering research in comparative genomics. When I sat with him today and asked him what it was like to receive such a distinction, he seemed genuinely surprised that I had even heard the news and immediately attributed his achievements to his unbelievable colleagues and students. He spoke very openly about his passion for research, love for MIT, outlook for the future of genomics, and pressures of living up to the hype that awards generate. Kellis was born in Greece, but moved with his family to France and eventually arrived in the U.S. in 1993. Manolis, his sister, and his brother were all accepted to MIT within nine months of each other. He says that MIT was the only school he applied to, and for him it was the obvious choice. At MIT, he felt that the sky was the limit and he could do whatever he wanted to do. But since his acceptance, he admits that his path has been partly determined by a series of coincidences. He chose to study Computer Science because it was an interesting, broad major that could open doors to any area. Manolis got his first, and only, UROP by "total chance." As he was walking through a corridor, he saw a friend who was on his way to a job orientation for the World Wide Web Consortium, led by Tim Berners-Lee (father of the Web). It sounded interesting so he tagged along and got chosen for one of the positions. He wrote a programmable WebCrawler for his project, but more importantly, he got an early start, which attracted companies and led to better opportunities. Without that UROP, he says, he "probably wouldn't be sitting here today". Manolis's interest in biology, and genetics in particular, also appears to be serendipitous. One day, he ran into a friend who happened to be reading a biology book that he himself owned. That friend opened his eyes to biology and introduced him to Eric Lander, the driving force behind the Human Genome Project, who eventually became his thesis advisor. Manolis says that seeing the genomic data - the string of A, T, G, and C's - was like seeing himself in the mirror. He became fascinated by the "code that makes us work" and "could never look back." Manolis continued his studies at MIT by getting a Masters in Engineering (M. Eng) in Electrical Engineering and Computer Science, and then entered the field of comparative genomics for his Ph.D. while the field was still in its infancy. Comparative genomics basically compares the genomes of different organisms to figure out what in the genome is important and how organisms might have evolved. For example, if the same string of A, T, G, and C's appears in dogs, rats, and humans at about the same place, then chances are that this string codes for something important that is worth keeping around for millions of years. So, Manolis compared the first four eukaryotic genomes, which all belonged to yeast, using a novel process to find genes and other pieces of DNA that determine when a gene is expressed. His research has received numerous awards and has been published seven times in the prestigious journal, Nature, in the past 3 years. Finally, Kellis accepted a faculty position at MIT because of the students, his love for academia, the sense of camaraderie, and the ability to be in the middle of everything. After all, he points out, where else can you teach at the #1 program in Computer Science, surrounded by some of the best biological and medical institutions in the world. He also gushes about his students, who he deems are "so brilliant." Kellis emphasizes that he has so much to learn from his students and loves the feeling that we are all sitting together at a round table, trying to understand science." In general, he finds MIT students to be intellectual, motivated, sincere, diverse, and down to earth. He also loves that he has always felt accepted by MIT professors and appreciates that he has been treated with the utmost respect since his freshman year. Few people have seen MIT from as many perspectives as Manolis Kellis: as an undergraduate, a graduate student, and a member of the faculty. I would also venture to say that few people understand what makes MIT unique as well as he does. He accredits the success of MIT to a group of people that are brilliant in their own ways but work together. The power of diversity is evidenced by the fact that a colony of genetically identical bacteria can be wiped out by a single antibiotic. In the same way, cloning is boring since the secret to survival lays in the mutations, the diversity. Kellis finally emphasized that in life there are no right answers, we must always be creative, grab opportunities when they appear, and accept that mistakes will happen. Innovator is well-suited to the language of MIT. http://www.masshightech.com/displayarticledetail.asp?art_id=67200&sec_id=42 Kellis, now an assistant professor at MIT, recalls that the family was living in Greece when his father decided to move the family to France. No one in the family could speak French. Indeed, none even knew where they were headed, which was a small city called Aix en Provence. "I had been at the head of my class in Greece, but suddenly I couldn't even speak the language of the school," said Kellis, 27. "I did math with a dictionary, first translating the problem from French to Greek before I could work on the problem." "Looking back, it was a great experience in having to adapt and acquire new skills. At the time it was tough." Kellis was honored recently in an event hosted by the Museum of Science. He was one of three young MIT professors declared "Young Innovators to Watch." Others honored were Chris Burge and Angelika Amon. Eric Lander, who heads the Broad Institute of genomic studies in Cambridge, introduced Kellis as one of the most promising researchers at the institute. Lander lauded Kellis for his ability to integrate computational paradigms to the complex tasks of deciphering DNA signals, understanding gene regulation and clarifying how genes evolve. Lander, founding director of the institute, said Kellis' work has the potential to help cure disease and also contribute to the understanding of natural history and evolution. The term "evolution" is a relevant one to Kellis, at least in terms of his education. After four years in France, he matriculated to the Lycee Francais in New York, where he added English to his linguistic repertoire. Never one to remain in one place, he enrolled at MIT. This decision was not as unexpected as some earlier ones: both his sister and brother were attending the Cambridge university. "I chose MIT because it's the best," Kellis said. "And the experience has turned out to be a great one." "What I love about the people - faculty and students - is the belief that nothing is impossible. People are always pushing the bounds of knowledge." After he received his undergraduate degree, he spent several years in San Francisco. He worked with Xerox PARC, a renowned research center for all things computer. Kellis says he worked on computational geometry, modular robotics and human motion understanding. Yet after several years there, he returned to Cambridge to seek his Ph.D. in bioinformatics. Like a good New Englander, he appears to have absorbed the credo that adversity, at least in terms of weather, can edify the man. Indeed, his web site contains the quote "Live in New York once, but leave it before it makes you hard. Live in California once, but leave before it makes you soft." Now again entrenched in Cambridge, Kellis is affiliated with both the Broad Institute and MIT's Computer Science and Artificial Intelligence Laboratory. Because bioinformatics is such a new field, he has the opportunity to become a pioneer as young scientists continue to add to the knowledge of the field. Though a career at MIT often results in professors leaving for high-tech startups, Kellis appears content to pursue his career in academia. "I just started an assistant professorship, and my goal is research and academic life," he said. "There might be consulting opportunities or business opportunities in the future, but at this point I am excited to be at an institution with so many possibilities for research." Finally, Kellis appears prepared to stay put. Genomic revelations from fly's family tree http://www.broad.mit.edu/cgi-bin/news/display_news.cgi?id=4083 In one of the first large-scale comparisons of multiple animal genomes, scientists at the Broad Institute of MIT and Harvard, the Computer Science and Artificial Intelligence Laboratory (CSAIL) at MIT, and many collaborating institutions, have analysed the genomes of twelve species of the fruit fly Drosophila to reveal insights on the evolution of genes and genomes and to discern the functional elements encoded in animal DNA. The work appears in the November 7 issue of Nature and in more than 40 accompanying papers in Genome Research and other journals. The method of comparing the genomes of multiple related species, fly or otherwise, not only reveals new insights into species evolution and identifies thousands of novel genes and other functional elements, but also provides a powerful tool for unravelling genome function that may help researchers unlock the secrets of our own genome. In these papers, the international consortium reported the genomes of ten newly sequenced Drosophila species, some very closely related and others less so, and their comparison to two previously sequenced flies including Drosophila melanogaster, one of the most powerful model organisms for the study of animal biology and evolution. The availability of the many Drosophila genomes has enabled a great deal of new insights about genome function and aided the study of how genomes have changed across evolutionary time. "Having the sequences of many closely related species allows us to study the evolutionary forces that have shaped the fruit fly's family tree, and to discover the working parts of the fly genome in a systematic way," said Manolis Kellis, associate member of the Broad Institute, assistant professor in MIT's CSAIL, and one of the consortium's project leaders. On one hand, the researchers studied the differences across species to help elucidate how evolution has shaped fly biology over millions of years. Their analysis revealed that while many attributes of Drosophila genomes are in fact conserved across multiple species, each species has novel features not seen in any other. In fact, only 77 percent of the approximately 13,700 protein-coding genes in D. melanogaster are shared with all of the other 11 species. For example, the genes involved in interactions with the environment and in reproduction showed signs of adaptive evolution, meaning that they likely provided some survival advantage to the organism. On the other hand, the researchers studied the similarities of the different species to help define the functional parts of the fly genome. The parts of a genome that are unchanged (conserved) are those that have been kept by evolution, and are thus likely to play crucial roles. Thus, genome comparison can reveal which regions of the genome are functional, based on the degree to which evolution has conserved them. "Focusing on the conserved part of the genome is a great way to discover what has been maintained by evolution," said Kellis. "Moreover, by looking more closely at the subtle patterns of mutation within conserved regions, we can predict the functional roles they play." Indeed, at the level of DNA, several combinations of letters, or nucleotides, may encode the same function, in the way that a storyteller can use different combinations of words to tell the same tale. For example, four different nucleotide combinations - GTT, GTC, GTA, and GTG - all encode the same protein building block, or amino acid. Thus, a change in the third letter would leave the amino acid unchanged, one example of how DNA changes can be tolerated while still preserving the function of the corresponding protein. Through these kinds of random mutations, evolution explores the space of possible nucleotide combinations that preserve function. This exploration produces unique patterns of genomic change, described by the researchers as "evolutionary signatures" that are specific to the function of that region of DNA. Protein-coding genes, for example, show frequent substitutions at every third nucleotide, due to the fact that one amino acid can be encoded by several nucleotide triplets. In contrast, some genes that don't encode proteins - so-called RNA genes - show changes that preserve the overall structure of RNA while tolerating changes in the genes' DNA sequence. Like codebreakers turning their knowledge of biology into computational algorithms, Kellis and his colleagues identified evolutionary signatures associated with a variety of roles in the genome: protein-coding genes, non-coding RNAs, microRNAs, and regulatory motifs. In each case, the researchers identified distinct evolutionary signatures associated with each function, based on the tolerated changes that still preserve that function. The researchers then used these evolutionary signatures to systematically identify the functional elements encoded in the fly genome, leading to hundreds of novel functional elements and many new insights on animal biology. The work allowed the discovery of 1,193 new sequences that encode proteins, the flagging of 414 regions that were mistakenly labelled as protein-coding genes, and corrections to hundreds of previously annotated protein-coding genes. This allowed the researchers to revise the catalogue of protein-coding genes for Drosophila melanogaster, with updates affecting 10% of all genes. The revision was confirmed through manual curation by scientists at the FlyBase consortium and through large-scale experimental validation led by the Berkeley Drosophila Genome Project. In addition, the researchers identified hundreds of new RNA genes and structures, new microRNA genes, and new DNA sequences involved in the control of gene expression during embryo development and environmental changes. The twelve genomes also allowed the prediction of very small regulatory targets in the genome, which can help piece together the first regulatory network for an animal genome without having to perform intense and expensive experiments. The work also led to many surprises. For example, the researchers found many protein-coding genes that defy the traditional rules of how the DNA code gets translated into protein. For example, 150 genes apparently bypass signals that would normally cause DNA to stop being translated, and other genes encode multiple proteins in a single RNA transcript. Other findings include surprising evidence that a single microRNA gene locus can produce up to four functional microRNAs, each with distinct functions. The team's analysis is the first time that such a diverse range of evolutionary signatures has been applied to identify the functional elements of a genome in a comprehensive way. "By comparing many closely related genomes, we were able to discover things we never thought were possible using one genome sequence alone," said Kellis. One intriguing possibility is that evolutionary signatures may even identify novel, yet unknown classes of functions. For example, although the fruit fly has been intensely studied for over a century, microRNAs were only discovered in the last decade, and are now known to play a central role in development. Many other classes of yet unknown functional elements may be hidden in the fly genome, and recognition of their common evolutionary properties may help lead to their discovery. The study of the 12 flies has immediate implications for the discovery of functional elements in the human genome. "We are now using similar methods to analyse 32 mammalian genomes, in order to help understand the human genome," Kellis explained. "We should be able to apply the methodology of evolutionary signatures to any group of closely related species." Fly consortium uncovers swarm of novel findings about genomic evolution, function http://www.eurekalert.org/pub_releases/2007-11/nhgr-sct110507.php Scientists compare 12 fruit fly genomes Fly consortium uncovers swarm of novel findings about genomic evolution, function An international research consortium of scientists, supported by the National Human Genome Research Institute (NHGRI), part of the National Institutes of Health (NIH), today announced publications comparing the genome sequences of 12 closely related fruit fly species, 10 of which were sequenced for the first time. The analyses identify thousands of novel genes and other functional elements in the insects' genomes, and describe how evolution has shaped the genomes of these important models for genetic research. "This remarkable scientific achievement underscores the value of sequencing and comparing many closely related species, especially those with great potential to enhance our understanding of fundamental biological processes," said Francis S. Collins, M.D., Ph.D., director of NHGRI. "Thanks to the consortium's hard work, scientists around the world now have a rich new source of genomic data that can be mined in many different ways and applied to other important model systems as well as humans." The fruit fly is one of the most important model organisms in genetic research. In studies dating back nearly a century, researchers used fruit flies to discover the basic rules of inheritance and to study how a single cell, the fertilized egg, develops into a whole animal. Because fruit flies are easy to work with in laboratory settings, they continue to be used as a model to study fundamental biological processes that occur in many living things, including humans. Although fruit flies have a genome that is 25 times smaller than the human genome, many of the flies' genes correspond to those in humans and control the same biological functions. In recent years, fruit fly research has led to discoveries related to the influence of genes on diseases, animal development, population genetics, cell biology, neurobiology, behavior, physiology and evolution. In papers published in the journal Nature, the Drosophila Comparative Genome Sequencing and Analysis Consortium compare the genome sequences of Drosophila melanogaster, which was published in 2000, and D. pseudoobscura, published in 2005, with the recently sequenced genomes of D. sechellia, D. simulans, D. yakuba, D. erecta, D. ananassae, D. persimilis, D. willistoni, D. mojavensis, D. virilis and D. grimshawi. In addition, two companion manuscripts in today's Nature were contributed by researchers from the Laboratory of Cellular and Developmental Biology of the National Institute of Diabetes and Digestive and Kidney Diseases, at NIH. The work was carried out by hundreds of scientists from more than 100 institutions in 16 countries. The sequencing of the 10 new genomes was led by Agencourt Bioscience Corp., Beverly, Mass. Other sequencing centers contributing to the sequencing were Washington University School of Medicine, St. Louis, Mo., the Broad Institute of MIT and Harvard, Cambridge, Mass., and the J. Craig Venter Institute, Rockville, Md. The sequencing centers were funded as part of NHGRI's Large-Scale Sequencing Research Network. To the average person, one fruit fly hovering around an overripe banana looks pretty much like any other. Researchers found that, at first glance, the genomes of the various types of fruit flies appear quite similar. However, a more detailed examination reveals that only 77 percent of the approximately 13,700 protein-coding genes in D. melanogaster are shared with all of the other 11 species. Scientists observed that different regions of the fruit fly genomes, including protein-coding genes and gene families, are evolving at different rates. For example, genes involved in taste and smell, detoxification and metabolism, sex and reproduction, and immunity and defense appear to be the most rapidly evolving in the fruit fly genomes. The findings suggest that these particular protein-coding genes likely evolve in the fruit fly genome as a result of adaptation to changing environments and sexual selection. For instance, the fruit fly species D. sechellia, whose population lives on the Seychelles islands in the Indian Ocean, is losing gustatory (taste) receptors approximately five times faster than other fruit fly species that generally encounter a more diverse set of foods than those available on an island. In a surprising finding, researchers found that the genes that produce selenoproteins appear to be absent in the D. willistoni genome. Selenoproteins are responsible for reducing excess amounts of the mineral selenium, an antioxidant found in a variety of food sources. Selenoproteins are present in all animals, including humans. D. willistoni appears to be the first animal known to lack these proteins. However, researchers suggest that D. willistoni may possibly encode selenoproteins in a different way, opening a new avenue for further research. A project leader and co-author for the studies, William M. Gelbart, Ph.D., of Harvard University in Cambridge, Mass., said "The availability of the 12 fruit fly genomes resulted in a dramatic increase in resolution allowing us to examine how evolution has fine-tuned biological processes. Our work shows that discovery power increases with the number of genomes available for comparison." More than 40 companion manuscripts with further detailed analyses are in current and forthcoming issues of Bioinformatics, BioMed Central (BMC) Bioinformatics, BMC Evolution Biology, BMC Genomics, Genetics, Genome Biology, Genome Research, Journal of Insect Science, Molecular Biology and Evolution, Nature Genetics, Public Library of Science (PLoS) Genetics, PLoS One, Proceedings of the National Academy of Sciences, and Trends in Genetics. In addition to their analyses aimed at gaining a better understanding of genomic evolution, consortium scientists used the 12 fruit fly genomes to identify thousands of new genes and other functional elements. This work will bolster efforts to find all functional elements in the reference genome sequence of D. melanogaster. "Comparing the 12 fruit fly genomes allowed us to recognize evolutionary signatures characteristic of each function. These signatures enabled us to distinguish and identify thousands of new functional elements." said Manolis Kellis, Ph.D., of the Massachusetts Institute of Technology in Cambridge, Mass., and a co-author of the Nature papers. Specifically, researchers used the evolutionary signals to discover 1,193 new protein-coding sequences and called into question 414 sequences previously reported as protein-coding genes in the D. melanogaster genome sequence. In addition, they found hundreds of novel functional elements across the 12 fruit fly genomes, including: non-protein coding genes; regulatory elements involved in the control of gene transcription; and DNA sequences that mediate the structure and dynamics of chromosomes. "Our analyses only represent a small portion of questions that can be answered in the context of these 12 species," said Andrew G. Clark, Ph.D., from Cornell University in Ithaca, N.Y., a co-author on the Nature papers. "Today's findings represent an important starting point for future research aimed at understanding the function of the genome features we discovered and their relevance to the human genome." Fruit fly genome provides evolutionary insight http://www.telegraph.co.uk/earth/main.jhtml?view=DETAILS&grid=&xml=/earth/2007/11/07/scifly107.xml Scientists have cracked the DNA code of a dozen different species of fruit fly, a tour de force that will lay bare new details of how evolution works. The study will shed light on human medicine too and has already revealed that earlier methods to find genes are flawed. The fruit fly is the world's favourite laboratory animal because many of their genes correspond to those in humans and control the same biological functions, a fact underlined how today's study was carried out by hundreds of scientists from over 100 institutions in 16 countries. "The evolution of the fruit fly is interesting in itself as, basically, they have been following us around the planet as we discard rotting fruit - they started off in Africa with us - now are everywhere," said Dr Ewan Birney of The European Bioinformatics Institute, Cambridge, who comments on the work in Nature. "Now these fruit flies, who have been our evolutionary companion as we left Africa, repay us by giving insights into their genome. Flies can remember things, they can get drunk," he said, listing some of the ways they will help human medicine. "Their evolution is particular interesting, especially in Hawaii where there are these weird super-sized flies," he said, adding that he expected the work will pave the way for similar comparisons of mammalian genomes. More than 40 manuscripts related to these studies are forthcoming, in addition to the papers published today in the journal Nature. The studies reveal new details of how genes are regulated- turned on and off - which is important to understand how relatively few genes - around 14,000 and 20,000 respectively, - can build an organism as complex as a fruit fly or a human being. Genes are messages written in DNA that control the manufacture of the proteins the build and operates our bodies. Today's papers also reveal major flaws in the way scientists identify genes. They found 1,193 new DNA sequences that encode proteins, 414 regions that were mistakenly labelled as protein-coding genes, and made corrections to hundreds of stretches of DNA previously thought to be protein-coding genes. The resulting corrections will affect 10% of all fruit fly genes. The scientists also learned certain genes appear to be evolving faster than others, such as the genes associated with smell and taste, sex and reproduction, and defences against disease. "Having the sequences of many closely related species allows us to study the evolutionary forces that have shaped the fruit fly's family tree," said Manolis Kellis, associate member of the Broad Institute, near Boston. "This remarkable scientific achievement underscores the value of sequencing and comparing many closely-related species, especially those with great potential to enhance our understanding of fundamental biological processes," said Dr Francis Collins, director of the US National Human Genome Research Institute, Maryland. "Scientists around the world now have a rich new source of genomic data that can be mined in many different ways and applied to other important model systems as well as humans." Surprises in fly genome http://www.the-scientist.com/news/home/53844/ Comparative genome sequencing of 12 Drosophila species reveals new genes, gene structures, and regulators Even after decades of genetic study, the Drosophila melanogaster genome still contains undiscovered genes and other genetic elements, according to a study in this week's Nature. By comparing evolutionary signatures in the genome sequences of 12 Drosophila species, the authors found new protein-coding, RNA, and microRNA genes, as well as gene regulators and targets. They also discovered that several unusual translation mechanisms -- including skipped stop codons and reading-frame shifts -- are more common than previously thought. Accompanying research papers in Nature this week present an overview of Drosophila genome evolution, as well as new findings in Drosophila sex chromosomes and sex-biased gene expression. Finding new protein-coding genes in an organism as well-studied as D. melanogaster is "an interesting surprise," said Elliott Margulies of the National Human Genome Research Institute in Rockville, Md., who was not involved in the work. Scientists led by four researchers -- Alexander Stark, Michael Lin, and Pouya Keradpour of the Broad Institute of MIT and Harvard in Cambridge, Mass., and Jakob Pedersen of the University of Copenhagen -- examined the 12 Drosophila sequences for evidence of regions that have been under natural selection. They scanned the genomes for unique evolutionary signatures associated with each type of genetic element. For example, conserved protein-coding regions usually show base changes that do not affect amino-acid sequence, while RNA genes allow mutations that preserve base-pairing interactions and microRNA genes show strong conservation only in certain parts of their sequences. This approach "kicks up a notch the kind of comparative genomics that you can do," Margulies said. While most previous comparative studiesonly allowed researchers to determine whether a given region went through selection, using these signatures identifies what type of element it likely is. The analysis predicted about 1,200 novel protein-coding exons in the Drosophila genome, corresponding to 150 new genes. Their results led to the revision of hundreds of gene transcription and translation models, which senior author Manolis Kellis of the Broad Institute said will be reflected in the next version of the annotated Drosophila genome at FlyBase. The authors found evidence of several unusual gene structures in the fly, such as stop-codon readthough, in which a stop codon is misread or skipped, and poly-cistronic genes, which code for two or more distinct proteins. They also found that the Drosophila genome contains several instances of "programmed" changes in the reading frame of translation, which alters how messenger RNA is read into protein. All of these discoveries were "really unexpected," Kellis told The Scientist. "Many protein-coding genes don't actually follow the rules you would expect them to follow." According to Ross Hardison of Pennsylvania State University in University Park, who was not involved in the work, these gene structures were thought to be very rare. "The importance of them becomes more obvious when you see multiple examples of them in a genome-wide study," he said. The comparative analyses uncovered new microRNA genes, RNA genes, and RNA structures involved in post-transcriptional processes such as messenger RNA editing and translational control. They also revealed many new gene regulators, including several found at higher levels in specific tissues than regulators already known to be important in these tissues. Their ability to add so much information to D. melanogaster annotated genome through comparative genomics shows "how powerful these methods are," Kellis said. Analyses Of 12 Fly Genomes Reveals New Insights On Genome Evolution And Regulation http://www.sciencedaily.com/releases/2007/11/071107160224.htm Genome Research is publishing a number of papers related to comparative analyses of twelve Drosophila (fly) genomes. The twelve fly genome project is unique in that the analyses of closely related species has allowed for a more complete and correct annotation of functional genes and regulatory elements in Drosophila melanogaster, a major model organism in genetics. With a life span of just weeks, the fruit fly has been an important model organism in genetic studies for decades and has helped researchers unravel the rules that govern inheritance. Though there are many differences between fruit flies and humans, the two also share many genes that regulate the same biological functions. Expanding universe of microRNAs http://www.sciencedaily.com/releases/2007/11/071107160224.htm MicroRNAs (miRNAs) are short RNA molecules encoded by plant and animal genomes that have garnered significant interest for their ability to regulate gene expression. A number of miRNAs have been discovered in recent years, however it is likely that many miRNAs have gone undetected. Two papers published in Genome Research utilize the twelve fly genomes to identify novel miRNAs, further refine the set of known miRNAs, and investigate the biology and origins of miRNA genes. In a study led by Dr. David Bartel, a combination of computational methods and high-throughput sequencing techniques identified new miRNAs conserved across the Drosophila species. "The new fly genomes enabled us to predict new miRNAs, 20 of which we experimentally confirmed, and the genome alignments enabled us to more accurately predict the evolutionarily conserved targets of these and other miRNAs," explains Bartel. While computational methods are important for identifying novel miRNAs, large-scale sequencing of small RNAs indicates that many miRNAs continue to evade prediction. "Most of the 59 novel miRNAs that we found were not predicted by us or by others," describes Bartel. "This illustrates the advantages of high-throughput sequencing of small RNAs, and the limitations of comparative sequence analysis for miRNA gene identification." In a related paper, a study led by Dr. Manolis Kellis utilized the twelve Drosophila genomes to computationally predict and experimentally validate novel miRNAs by defining the structural and evolutionary properties of known miRNAs. Classification of newly identified miRNAs has revealed greater diversity in the regulation gene expression by miRNAs, with increased potential for combinatorial regulation, and provided new insights on miRNA biogenesis and function. "We learned that both arms of a miRNA hairpin can produce functional miRNAs, which sometimes work cooperatively to target a common pathway," explains Kellis. The combination of comparative and experimental analyses by both groups also provided novel evidence for emergent gene function, deriving from the portion of the miRNA hairpin previously believed to be discarded, and the strand of the DNA previously not thought to produce a miRNA. Revisiting D. melanogaster http://www.sciencedaily.com/releases/2007/11/071107160224.htm Drosophila melanogaster is one of the most intensely studied model organisms in biology. Numerous studies over the years have defined nearly 14,000 protein-coding genes by experimental and computational methods, however these methods are likely to have produced erroneous annotations or may be missing other annotations. In order to assess the D. melanogaster protein-coding gene catalog, a group of researchers led by Dr. Manolis Kellis identified evolutionarily signatures of protein-coding genes by comparative analysis of the twelve fly genomes. This strategy was then applied to evaluation of the current catalog and identification of genes that have escaped annotation. The study led to the discovery of hundreds of new genes, refined existing genes, and concluded that greater than 10% of the protein-coding gene annotations requires refinement. Additionally, the work revealed abundant unusual gene structures. "We have learned that many brain-expressed proteins may be undergoing post-transcriptional changes by stop-codon read-through," explains Kellis. "We found 149 genes for which a conserved stop codon is followed by strong evidence of protein-coding selection for up to hundreds of amino acids, suggesting a new mechanism for post-transcriptional regulation in animal genomes." The researchers also report additional widespread evidence suggesting several diverse mechanisms of post-transcriptional regulation for protein-coding genes. Scientists compare 12 fruit fly genomes http://news.xinhuanet.com/english/2007-11/08/content_7029878.htm WASHINGTON, Nov. 7 (Xinhua) -- An international research consortium of scientists announced on Wednesday their publications comparing the genome sequences of 12 closely related fruit fly species, 10 of which were sequenced for the first time. The analyses identify thousands of new genes and other functional elements in the insects' genomes, and describe how evolution has shaped the genomes of these important models for genetic research. The work was carried out by hundreds of scientists from more than 100 institutions in 16 countries. In papers published in the journal Nature, the consortium compare the genome sequences of Drosophila melanogaster, which was published in 2000, and D. pseudoobscura, published in 2005, with the recently sequenced genomes of 10 fruit fly species. Researchers found that, at first glance, the genomes of the various types of fruit flies appear quite similar. However, a more detailed examination reveals that only 77 percent of the approximately 13,700 protein-coding genes in D. melanogaster are shared with all of the other 11 species. Scientists observed that different regions of the fruit fly genomes, including protein-coding genes and gene families, are evolving at different rates. For example, genes involved in taste and smell, detoxification and metabolism, sex and reproduction, and immunity and defense appear to be the most rapidly evolving in the fruit fly genomes. The findings suggest that these particular protein-coding genes likely evolve in the fruit fly genome as a result of adaptation to changing environments and sexual selection. "Scientists around the world now have a rich new source of genomic data that can be mined in many different ways and applied to other important model systems as well as humans," said Francis Collins, director of the U.S. National Human Genome Research Institute, which supported the project. Making a buzz: Gene study on fruitflies sheds light on evolution http://afp.google.com/article/ALeqM5iEWRTP0p6DJt8xGPrESLohY4vlDg Lab sleuths said they had laid bare the complete genetic code of a family of fruitflies, enabling the world's first comparison of the genomes of a dozen closely-related species. The achievement opens a window onto key evolutionary processes and may one day serve as a model for understanding why we humans and our nearest primate cousins are so close yet also so different, they believe. In studies to be published on Thursday in the British journal Nature, around 150 scientists around the globe added 10 fully-sequenced genomes of the fruitfly Drosophila to two that had already been decoded. They also began to mine the treasure trove of data, publishing analyses touching on two cornerstones of evolutionary theory -- the principles of positive and negative selection. Positive selection means genetic mutations that spread through a species because they provide an advantage in the struggle to survive. Negative selection means genetic characteristics that are forced out of a species because they are an encumbrance to survival. By looking across a broad family, researchers can spot a mutation that has been favoured as it confers an evolutionary advantage on a specific species -- such as a change in the immune system -- or a mutation that has been weeded out. They can also identify genetic code that has remained unchanged, or conserved, because of its enduring usefulness to the species. Histone proteins, which determine how DNA is packed inside cell nuclei, have barely changed over the 60 million years going back to the single common ancestor from which all the Drosophila species eventually emerged, the investigators found. "Once evolution figures how to make something like that work, it does not change easily," said David Rand of Brown University, whose laboratory worked on sequencing mitochondrial DNA from all 12 fruitfly genomes. Only 77 percent of the approximately 13,700 protein-coding genes are shared by all 12 species, the consortium says. The humble fruitfly, especially the main species, Drosophila melanogaster, has long been used to probe the biology of multicellular organisms. It is familiar to many as the barely visible objects of school-age experiments on the mechanics of heredity. Over the last decade, however, geneticists have gradually shunted the harmless flies aside in favor of worms and especially mice, which offer better models for studying the relationship between genes and human disease. But the newly sequenced "Drosophila Dozen," which range from the tiny D. simulans to the relatively giant D. grimshawi of Hawaii and the red-eyed D. yakuba of the African savannah, are sure to rekindle interest in the winged beasts. "The 12 Drosophila genomes give us an unprecedented opportunity to understand evolutionary adaptation right down at the genetic level," said Brown researcher Kristi Montooth. "If we want to understand how the fly that lives on the savannah is different from the fly that lives in the desert, we can trace physiological differences back to specific genes." A novel approach to DNA analysis http://www.technologyreview.com/Biotech/19689/ In a milestone for the emerging field of comparative genomics, an international team of scientists has carried out a comparative analysis of the genome sequences of 12 different species of fruit flies. Not only did the researchers uncover patterns in the way that genes evolve as species adapt to different environments, but they also developed a new way of identifying the functional elements of the genome--a discovery with potentially far-reaching consequences. For more than a hundred years, the fruit-fly species Drosophila melanogaster has been instrumental in the study of genetics, developmental biology, and animal behavior. Because a significant number of human genes have fruit-fly analogues, researchers have also used the insect to study many human diseases, including cancer, diabetes, and neurodegenerative disorders such as Alzheimer's. In 2000, scientists published the genome sequence for D. melanogaster; the sequence of a second fruit-fly species followed several years later. There are 1,500 species of fruit flies, however, and they vary in appearance, behavior, and habitat. To fully understand the fruit-fly genome and how it has evolved, a consortium of more than a hundred labs around the world sequenced an additional 10 species and compared all 12 sequences. The group details its findings in two reports published in the November 8 issue of Nature. "If you want to get a crystal-clear picture of how genes influence what an animal will look like, what it will eat, what behavior it will exhibit, this is a completely unparalleled resource for doing that," says Leslie Vosshall, a neurogeneticist at Rockefeller University, in New York. The researchers selected species from all over the world--from Africa, Asia, the Americas, and the Pacific Islands. Some species are widespread and feed on a range of foods, whereas others are more limited. For instance, one species lives only on the Seychelles islands off the east coast of Africa and eats only one kind of fruit. In one of the papers, a team led by Manolis Kellis, a computational biologist at MIT, compared the 12 sequences in order to identify all the functional elements in the fruit-fly genome. These include not only genes that code for proteins, but also sequences that help regulate gene expression by, for instance, encoding small RNA molecules that bind to other parts of the genome. To find these elements, researchers typically look for sequences that are common, and therefore highly conserved, among different genomes. "The basic premise of comparative genomics is that if something is conserved over millions of years in a dozen species, it's likely to do something useful," says Kellis. But Kellis and his colleagues were also seeking an alternative strategy. They figured that by looking only for sequences that have remained roughly the same, they would miss a large number of functional elements. For instance, protein-coding genes can undergo extensive changes and yet retain their critical functions. By looking at all 12 genomes, the team found that each type of functional element changes in characteristic ways over time, and those patterns of change serve as evolutionary signatures. For instance, a series of three-letter DNA sequences in which the first two letters are always conserved but the third one changes is likely to be a protein-coding gene, says Kellis. So the researchers designed computer algorithms to mine the sequence data and find the evolutionary signatures for each type of functional element. "This allowed us to find things that we would never have expected to find just by looking at a single genome," says Kellis. Kellis's team found thousands of previously unidentified functional elements, including 150 protein-coding genes and more than a hundred microRNA genes. (MicroRNAs are short segments of RNA that silence genes by binding to specific sites in the genome.) The researchers also found that some genes, during their translation into proteins, ignore certain instructions and, as a result, acquire bits of protein encoded by other genes. "This is an entirely new mechanism," says Kellis, adding that his group has since found evidence of this mechanism in the human genome as well. The second Nature paper describes research led by Andrew Clark, a population geneticist at Cornell University, who looked at known genes to see how they vary from one species to another and how they evolve, acquiring new functions as species adapt to their changing environments. Genes involved in the immune system, for instance, appear to evolve more rapidly than genes in the rest of the genome. The same was true for genes that regulate insecticide resistance. Taste and smell receptor genes also undergo frequent changes. When the researchers compared species of flies that are generalists with those that have more specialized food preferences, they found that the specialists lose genes for different taste receptors at a much higher rate than the generalists do. "How you smell the world influences how you eat, and this will tell us an enormous amount about how genes that encode for smell and taste influence behavior," says Vosshall. The studies of the 12 fruit-fly genomes will no doubt help scientists better understand the human genome, says Kellis. Not only do fruit flies and humans have so many genes in common, but now researchers have a systematic way of interpreting genomes that could lead to the discovery of entirely new kinds of functional elements, he says. A fruity dozen: sequencing effort nets many fly genomes http://origin.arstechnica.com/journals/science.ars/2007/11/07/afruity-dozen-sequencing-effort-nets-many-fly-genomes Normally, our reports focus on one or two papers that mark a major or intriguing scientific milestone. Today is an exceptionplay a huge role in evolution. In any species' lineage, nearly half the gene families will change in size. Somewhere in the genome, a family member will be gained or lost every 60,000 years. The specialist, sechellia, lost more than most, as its simplified lifestyle has allowed it to forgo many metabolic activities and defenses against toxins. In contrast to this rampant reuse of existing genes, new genes appeared rarely. The researchers detected only 44 new genes that were clearly not the result of horizontal gene transfer. Most of them were short, intron-free, and involved in sex and reproduction. The massive data set also gives us greater perspective on the rare and unusual. Many large, conserved protein coding regions have stop codes in the middle of them. The authors that identified them suggest that 123 are cases where a single transcript codes for more than one protein, something that was once thought to only happen in bacteria. Another 150 or so seem to have the stop codon edited out by enzymatic alteration of the RNA (common in some single-celled Eukaryotes, but not recognized to occur widely in animals). I'm only scratching the surface of a couple of the publications, but it seems that anyone interested in biology will find something compelling somewhere in the blizzard of publications coming out of these genomes. And get ready for something even bigger: a similar effort is already well underway to sequence an equivalent group of mammals. Scientists complete DNA sequencing and analysis of multiple fruit fly genomes http://www.sciencecentric.com/news/07110720.htm Copy of Broad Press Release Fruit Fly Blitz Shows the Power of Comparative Genomics http://www.sciencemag.org/cgi/content/summary/318/5852/903 A consortium of about 250 researchers have done a 12-way comparison to track the evolution of genes, regulatory regions, entire pathways, and cellular processes. Having these patterns in hand makes it easier to spot similar features in the genomes of other species, including humans, researchers report in more than 40 research papers in the 8 November issue of Nature and in other journals. "This work has really increased the sophistication of what we can learn from comparative sequence analysis," says genomicist Elliott H. Margulies of the National Human Genome Research Institute (NHGRI) in Bethesda, Maryland. As project co-leader Michael Eisen of Lawrence Berkeley National Laboratory in California points out, the comparison "allows you to map where [genetic] changes occur along the tree, and that allows you to study the process of evolution, not just the product." Manolis Kellis of the Broad Institute in Cambridge, Massachusetts, led an assessment of how each type of gene or regulatory region changed--or didn't change--from one species to the next, revealing specific evolutionary patterns, or signatures. Kellis and others have incorporated those telltale patterns into software to look for the same patterns in other species to pinpoint each type of DNA. "This allows us to assign function" to some regions "through computation alone," says Margulies. Based on a common pattern of insertions, deletions, base usage, and substitutions, Kellis and his colleagues detected 192 undiscovered protein-coding genes as well as 150 that do not follow standard rules. Typically, proteincoding genes have a "stop" sequence that signals the end of the gene. But in these 150 cases, protein-coding sequences extended beyond the "stop." "It's always a little humbling that the assumptions we are taught in school do not apply across all genes," says Ewan Birney of the EMBL European Bioinformatics Institute in Hinxton, U.K. With these new tools, which are particularly useful for recognizing regulatory DNA, Kellis and his colleagues have pieced together a fruit fly gene regulatory network that incorporates 81 microRNAs and 67 transcription factors. "The methodology and principles are absolutely general, and they are applicable to any genome," says Kellis. Others say that the model still needs refining to reconcile it with experimental results. But geneticist Rama Singh of McMaster University in Hamilton, Canada, is quite pleased with this beginning. Because many fruit fly and human genes are equivalent, the network "is going to tell us a lot about humans," he predicts. The analysis bodes well for the utility of bird, marsupial, and reptile sequences in analyzing the human genome. It also argues for sequencing and comparing all the primates, says Birney: "The take-home message is that there are a lot of clear wins from doing this sort of evolutionary genomics." UNM Graduate Student is Co-author of Paper in Nature http://www.unm.edu/~market/cgi-bin/archives/002407.html UNM Graduate Student Sushmita Roy spent last summer at the Massachusetts Institute of Technology in the lab of Manolis Kellis, an assistant professor of Electrical Engineering and Computer Science. He specializes in developing computational algorithms for decoding the information present in the genomes of organisms. As part of her internship, Roy played a small part in a large project analyzing the genomes of 12 fly species. The paper describing the project and its results was released this week in the journal "Nature." In her summer project, she analyzed statistical properties of the fly regulatory network, computationally predicted by Kellis' lab, with nodes representing genes and edges representing regulatory control exercised by a "regulator" gene on a "regulate" gene. This led to the identification of network nodes with different types of connectivity. Nodes with high-connectivity were themselves regulators controlling important events in the growth and development of flies. Roy says the edges in the network also had non-random properties. Edges had a higher chance of existing between genes functioning in the same fly tissue, rather than in different tissues. The identification of these statistical properties helped the researchers to clarify the biological significance of the predicted regulatory network of developing flies, which can provide insight into important developmental events in higher organisms. The title of the journal article is "Discovery of Functional Elements in 12 Drosophila Genomes Using Evolutionary Signatures." Roy is listed as one of the co-authors on the article. Roy is working on her Ph.D. in Computer Science applying statistical algorithms to understand living systems. Her advisors, Assistant Professor of Computer Science Terran Lane and Professor of Biology Margaret Werner-Washburne are guiding her efforts to apply statistical algorithms to understand living systems. Her internship in the MIT summer program was sponsored by the Program in Interdisciplinary Biological and Biomedical Sciences (PIBBS) at UNM and (Howard Hughes Medical Institute (HHMI) Interfaces program and was arranged by Bruce Birren, director of the Microbial Sequencing Center and co-director of the Genome Sequencing and Analysis program at the Broad Institute at the MIT and UNM Biology Professor Margaret Werner-Washburne. Genome Analysis of Twelve Drosophila Species http://www.mcb.harvard.edu/NewsEvents/News/Gelbart2.html On Thursday, November 8, there was a birth announcement that has been two years in the making: the initial publications on the comparative genome analysis of the entire DNA sequences of 12 species of fruit fly (genus Drosophila). The announcement will include two main papers in Nature describing the community effort. One paper (Kellis et al.) focuses on the identification of evolutionary signatures for several different classes of functional elements within the Drosophila melanogaster genome. The other (Drosophila 12 Genomes Consortium) focuses on understanding gene and genome evolution using whole genome datasets. In addition, publication of these papers has been coordinated with the near-simultaneous publication of more than 40 companion papers in several different journals, notably Nature, GENETICS, Genome Research, PLoS, BMC, Molecular Biology & Evolution, Genome Biology and others. This work comprises the research efforts of literally hundreds of scientists for the past 2-3 years. The Harvard MCB FlyBase group (Bill Gelbart, PI, Lynn Crosby, Bev Matthews, Andy Schroeder, Susan St. Pierre, Sian Gramates, Rob Kulathinal, Margo Roark, Ken Wiley, Jr., Kyl Myrick, Jerry Antone, AJ Bhutkar, Susan Russo and Peili Zhang) has been an integral part of the analysis coordination of these efforts. Several members of this group are co-authors on the main papers, as well as three companion papers that are in press and two others that are still in preparation. A number of surprising findings, only revealed through this kind of comparative genome analysis, were discovered. "First, more than 100 genes are expressed in ways that violate typical genetic code dogma, such as stop codon readthrough and shifting of reading frame in the middle of a translated segment", says Dr. Bill Gelbart. Secondly, "a species that, during its evolution, has dropped the use of selenocysteine (the so-called 21st amino acid) during translation (Drosophila willistoni)" was discovered. In addition to these findings, the initial analyses lay the foundation for future studies that will dissect the functional elements of the genomes in exquisite detail and that will help understand how ecological specialization is reflected in genome evolution. Finally, this work presents a model for selecting a cluster of closely related species for whole genome sequencing, allowing better understanding of the gene products and other functional elements encoded by the genome of a species of biological or practical importance. Kellis, MIT team announce significant findings in fly genome studies. http://www.eecs.mit.edu/cgi-bin/announcements.cgi?page=2007/data/345.dat Manolis Kellis, the Karl Van Tassel Career Development Assistant Professor of Electrical Engineering and Computer Science at MIT, and also affiliated with the Computer Science and Artificial Intelligence Lab, (CSAIL) and the Broad Institute of MIT and Harvard, has announced the culmination of several years of efforts to describe the sequencing and analysis of 12 Drosophila genomes. The work, a large-scale project comprising a diverse interdisciplinary team of scientists and co-led by the group at MIT, and also including scientists at the Whitehead Institute for Biomedical Research and the Harvard Department of Molecular and Cellular Biology, uncovers the functional elements encoded in the fruit fly genome as well as their evolutionary dynamics. The work resulted in many novel findings about the biology of animal genomes, and the computational approaches used promise in this work is that the approach may also to help unlock the secrets of many other genomes, including those of the human genome. This work appears in the Nov. 8 issue of Nature and in more than 40 accompanying papers in Genome Research and other journals. The group at MIT, including the Broad Institute and the Whitehead Institute for Biomedical Research, led the discovery effort, the first of its kind and scale, ranging across protein-coding genes, RNA genes, microRNAs, regulatory motifs, and regulatory networks. By comparing the 12 species, they Kellis and his colleagues were able to discover thousands of new genes and other functional elements in the fruit fly, learning a tremendous amount about the animal biologygenomes of animal genomes, and revealing new insights into their functioning and regulation, and the scaling of comparative discovery power with many species. In particular, the analysis showed that some protein-coding genes defy the traditional rules of protein translation, reading through stop codons for sometimes hundreds of amino-acids, and some microRNA genes can produce many functional products from a single regions, encoded in overlapping ways. Kellis reports: "The results have major implications on the understanding of the human genome, and the our team at MIT is now leading the an effort on the to discover the functional elements in the human genome analysis of by comparing 32 eutherian mammals to understand the human. We are already now using similar methods to analyze 32 mammalian genomesin the human, in order to help understand the human genomecombined with large-scale experiments to study tissue-specificity, cell differentiation, and epigenomics," Kellis explains. "We should be able to apply tThe methodology of using evolutionary signatures to discover functional elements is general, and should be applicable to any group of closely related species." Computational comparison of multiple Drosophila genomes proves to be a powerful research tool. http://www.csail.mit.edu/events/news/2007/drosophila.html CSAIL's Computational Biology Group led by Manolis Kellis co-led one of the first large-scale comparisons of multiple animal genomes. Results of the project will appear in four papers in Nature, and 40 companion papers in Genome Research, Genetics, Nature Genetics, and other journals. One of the unique aspects of this project is that it was led by computational scientists, working with dozens of experimental labs to validate and test hypotheses. "Our group at MIT led the discovery effort, the first of its kind and scale, ranging across protein-coding genes, RNA genes, microRNAs, regulatory motifs, and regulatory networks," says CSAIL PI and Broad Institue Associate Member, Manolis Kellis. "By comparing 12 species the fruit fly Drosophila, we were able to discover a tremendous amount about the biology of animal genomes and reveal new insights into their functioning and regulation. The technique of comparing genomes of multiple related species also provides a powerful methodology that could help researchers in the study of other genomes, including that of humans." Massive Project Reveals Shortcomings Of Modern Genome Analysis http://www.sciencedaily.com/releases/2007/11/071107160222.htm The sequencing and comparison of 12 fruit fly genomes -- the result of a massive collaboration of hundreds of scientists from more than 100 institutions in 16 countries -- has thrust forward researchers' understanding of fruit flies, a popular animal model in science. But even human genome biologists may want to take note: The project also has revealed considerable flaws in the way they identify genes. "We've made huge progress in recent years with many genomes, including humans, but a lot of the problems can't be solved by simply dumping data into a computer and having truth and light come out the other end," said Indiana University Bloomington biologist Thomas Kaufman, who co-led the project. "One of the things we've learned from this project is that when you compare a lot of different but related genomes, you are more likely to see the genes that are buried in all that A-C-T-G mush." Two current papers in Nature separately report the results of the four-year genome project and use the data to draw some conclusions about the fruit fly genus Drosophila, particularly its star species, the human nuisance Drosophila melanogaster. Among the papers' conclusions is the idea that resolving any individual species' genome is greatly enhanced when related genomes are compared to it. "This remarkable scientific achievement underscores the value of sequencing and comparing many closely-related species, especially those with great potential to enhance our understanding of fundamental biological processes," said Francis S. Collins, director of NHGRI. "Thanks to the consortium's hard work, scientists around the world now have a rich new source of genomic data that can be mined in many different ways and applied to other important model systems as well as humans." The consortium purposely chose a wide variety of fruit flies for study, guessing correctly that both gene similarities and differences among the 12 species would be easier to identify. Some of the Drosophila species the scientists studied are closely related to D. melanogaster, some not. Some of the flies fulfill very specialized ecological niches, such as D. sechellia, which has evolved a unique ability to detoxify the fruit of the Seychelles' noni tree. The other 10 species the consortium examined were D. pseudoobscura, D. simulans, D. yakuba, D. erecta, D. ananassae, D. persimilis, D. willistoni, D. virilis, D. grimshawi, and the cactus-loving D. mojavensis. D. melanogaster's genome was published in 2000 and D. pseudoobscura's genome was published in 2005. The other genomes are newly published. In comparing the 12 genomes, the scientists found 1,193 new protein-coding genes and hundreds of new functional elements, including regulatory sequences that determine how quickly genes are expressed, and genes that encode functional RNAs such as small nuclear RNAs. They also learned certain genes appear to be evolving faster than others, such as the genes associated with smell and taste, sex and reproduction, and defenses against pathogens. The Drosophila 12 Genomes Consortium found that D. melanogaster shares about 77 percent of its genes with the other 11 species they studied. The scientists also found errors in about 3 percent of previously sequenced D. melanogaster protein-coding genes, correcting 414 gene sequences on record. A vexing problem for genomicists is finding genes and other important DNA sequences in heterochromatin, tightly packed areas of chromosomes presumed to experience little expression. Heterochromatin is common in animal genomes. "The heterochromatin is very hard to analyze," Kaufman said. "Studies show heterochromatin changes the most. It's full of intermediate- and full-repeat sequences. And there are genes buried in this stuff." The conventions for locating the genes that encode proteins are pretty well established. The lingering problem for genomics biologists is locating genes whose parts are interrupted repeatedly, as well as locating genes that do not code for proteins. By comparing a huge number of genomes, these sorts of genes are relatively easy to locate. Genes that do important things for cells or tissues are more likely to be "conserved" over time; that is, they don't change much despite millions of years of mutations. Although fruit flies have a genome that is 25 times smaller than the human genome, many of the flies' genes correspond to those in humans and control the same biological functions. In recent years, fruit fly research has led to discoveries related to the influence of genes on diseases, animal development, population genetics, cell biology, neurobiology, behavior, physiology and evolution. One of the companion pieces accompanying this week's Nature papers was written by IUB computational biologist Matthew Hahn. Hahn reports in PLoS Genetics that although all 12 Drosophila species have about the same number of genes (14,000), the genomes are more dynamic than one might expect. "The highest turnover in gene number occurs in genes involved in sex and reproduction," Hahn said. "Our results demonstrate that the apparent stasis in total gene number among species has masked rapid turnover in individual gene gain and loss. It is likely that this evolutionary revolving door has played a large role in shaping the morphological, physiological, and metabolic differences among species. This is the reason the 12 species only share 77 percent of their genes." Kaufman co-founded the project with Cornell University's Andrew Clark, North Carolina State University's Gregory Gibson, Howard Hughes Medical Institute's Eugene Myers, University of California Berkeley's Patrick O'Grady, and University of Arizona's Therese Markow. FlyBase, a joint project of IU Bloomington, UC Berkeley, and Cambridge University, helped researchers access and study the 12 sequenced Drosophila genomes. Kaufman also directs the National Institutes of Health-funded Drosophila Genome Resource Center. Sequencing work was handled by research staff at the Baylor College of Medicine, the Broad Institute of M.I.T. and Harvard University, the Washington University School of Medicine, Agencourt Bioscience Corp., and the J. Craig Venter Science Institute. Decoding effort reveal fly species' DNA http://www.news.harvard.edu/gazette/2007/11.08/09-fruitflies.html Collaboration of researchers from 16 nations sequences fruit fly genomes An enormous effort to decode the DNA of one of science's most important laboratory animals - the fruit fly - ended in success this week as a collaboration of researchers from 16 nations announced the sequencing of 10 fly species' genomes. The research allows the extraordinary side-by-side comparison of the DNA of 12 species of fruit flies - two had already been decoded - as scientists search to understand the workings of individual genes and how those genes translate into specific physical characteristics. Already the research has produced results. Scientists involved in the collaboration also announced the findings of their initial analysis: the discovery of thousands of new genes and other functional elements such as DNA segments responsible for turning genes on and off. "The availability of the 12 fruit fly genomes resulted in a dramatic increase in resolution, allowing us to examine how evolution has fine-tuned biological processes. Our work shows that discovery power increases with the number of genomes available for comparison," said Harvard Professor of Molecular and Cellular Biology William Gelbart, one of the project's leaders. The results were announced in a series of papers in the Nov. 8 issue of the journal Nature. The work, conducted by hundreds of researchers at more than 100 institutions, was supported by the National Institutes of Health's National Human Genome Research Institute. "This remarkable scientific achievement underscores the value of sequencing and comparing many closely related species, especially those with great potential to enhance our understanding of fundamental biological processes," said National Human Genome Research Institute Director Francis Collins. "Scientists around the world now have a rich new source of genomic data that can be mined in many different ways and applied to other important model systems as well as humans." With a life span of just weeks, the fruit fly has been an important model organism in genetic studies for decades and has helped researchers unravel the rules that govern inheritance. Though there are many differences between fruit flies and humans, the two also share many genes that regulate the same biological functions. The dozen fruit flies now sequenced all belong to the genus Drosophila, which has about 2,500 different species. Though some may think there's little difference between fruit fly types, Gelbart said the genetic variation between fruit fly species is as large as that found among mammals. Fruit flies are adapted to life in a wide variety of conditions, from the desert to the rain forest, and have a wide range of physical traits. With researchers spanning many institutions around the world, Gelbart said the project, officially called the Drosophila Comparative Genome Sequencing and Analysis Consortium, was at times a challenge to keep moving forward. Gelbart accomplished the feat together with eight other project leaders from the Broad Institute of MIT and Harvard, Cornell University, the University of California, Berkeley, the Lawrence Berkeley National Laboratory, the Agencourt Bioscience Corp., the University of Manchester, the National Institutes of Health, the University of Arizona, Indiana University, and the Computer Science and Artificial Intelligence Laboratory in Cambridge, Mass. Among the findings from their side-by-side analysis of the dozen fruit fly genomes, researchers were able to determine that some genes are evolving faster than others. Genes involved in sex, taste, smell, detoxification, and metabolism seem to be evolving most rapidly. One example showed that a fly native to the Seychelles islands, with a limited universe of foods, is losing taste receptors much faster than other species. Researchers also discovered the first known animal to lack genes to produce selenoproteins, a type of protein needed to get rid of excess selenium in the body, in the fruit fly species Drosophila willistoni. The side-by-side comparison not only allowed the discovery of new genetic elements, it also allowed the correction of past errors. The results call into question more than 400 genes previously thought to encode proteins in the first species to be sequenced, Drosophila melanogaster. The work, which will be available to other researchers, not only opens up numerous new avenues for inquiry, it creates an enormous bank of information available to future scientists probing life's mysteries. "Like most science, it raises more questions than it answers, but that's OK," Gelbart said. Making Sense of Anti-Sense MicroRNAs http://www.innovations-report.de/html/berichte/biowissenschaften_chemie/bericht-100712.html Three independent papers in the January 1st issue of G&D report on the discovery of a bidirectionally transcribed microRNA (miRNA) locus in Drosophila. The studies from Drs. Alexander Stark and Manolis Kellis (MIT) and colleagues, and from Dr. Eric Lai (MSKCC) and colleagues, both reveal that antisense transcription of the Hox miRNA locus, miR-iab-4, generates the novel miRNA precursor mir-iab-8, which is processed into active regulatory RNAs. When ectopically expressed, mir-iab-8 generates homeotic phenotypes via direct repression of Hox gene targets. The paper from Dr. Welcome Bender (Harvard Medical School) demonstrates that knock out of miR-iab-4 reveals the existence of a miRNA transcribed from the opposite strand. Furthermore, the loss of the antisense miRNA causes subtle derepression of a hox gene and results in sterility of the mutant flies. The identification of additional antisense miRNAs in Drosophila and mammals suggests this as a mechanism that may contribute to the diversification of miRNA function. MIT reports new twist in microRNA biology http://web.mit.edu/newsoffice/2008/micro-rna-0109.html Computational biology group identifies new mechanism of gene regulation MIT scientists have found a new way that DNA can carry out its work that is about as surprising as discovering that a mold used to cast a metal tool can also serve as a tool itself, with two complementary shapes each showing distinct functional roles. Professor Manolis Kellis and postdoctoral research fellow Alexander Stark report in the January 1 issue of the journal Genes & Development that in certain DNA sequences, both strands of a DNA segment can perform useful functions, each encoding a distinct molecule that helps control cell functions. DNA works by complementarity: paired DNA strands serve as a template for each other during DNA replication, and ordinarily only a single DNA strand serves as a template to produce RNA strands, which then go on to produce proteins. The process is similar to the way each bump or dent in a mold is paired with a corresponding dent or bump in the resulting molded object. While many RNAs are eventually translated into proteins with specific functions, some RNA molecules instead act directly, carrying out roles inside the cell. Certain RNA genes, known as microRNAs, have been shown to play important regulatory roles in the cell, often coordinating important events during the development of the embryo. These microRNAs fold into relatively simple hairpin structures, with two stretches of near-perfect complementary sequence folding back onto each other. One of the two 'arms' of a hairpin is then processed into a mature microRNA. The surprising discovery is that for some microRNA genes, both DNA strands, instead of just one, encode RNA, and both resulting microRNAs fold into hairpins that are processed into mature microRNAs. In other words, both the tool and its mold appear to be functional. Kellis and Stark found two such microRNA pairs in the fruit fly, and eight more such pairs in the mouse. The idea that there could be such dual-function strands, where both DNA strands encode functional RNA products, "had never even been hypothesized," Kellis says. But followup work confirmed that they did indeed function in this way. The work suggests that other such unexpected pairings, with both DNA strands encoding important functions, may also exist in a variety of species. This discovery builds on a similar, earlier surprising finding about microRNA regulation. In December, Stark and Kellis reported that both arms of a single microRNA hairpin can also produce distinct, functional microRNAs, with distinct targets. Together, these two findings suggest that a single gene can encode as many as four different functions - one hairpin from each of the two DNA strands, and then one microRNA from each of the two arms of each hairpin. These recent papers are the latest example of the power of using computational tools to investigate the genomes of multiple species, known as comparative genomics. The Kellis group has used this approach to discover protein-coding genes, RNAs, microRNAs, regulatory motifs, and targets of individual regulators in diverse organisms ranging from yeast and fruit flies to mouse and human. "This represents a new phase in genomics-making biological discoveries sitting not at the lab bench, but at the computer terminal," Kellis says. Kellis is the Karl Van Tassel Career Development Assistant Professor in the Department of Electrical Engineering and Computer Science and an associate member of the Broad Institute. He grew up in Greece and France and earned his B.S., M.Eng., and Ph.D. from MIT, and he was appointed to the faculty here in 2004. At 30, he has already earned numerous awards and accolades, including a place on the list of the 35 top innovators under 35 by Technology Review magazine in 2006. Kellis' work is supported in part by grants from the National Institues of Health and the National Science Foundation. Alex Stark is supported by a Human Frontier Science Program fellowship. Human Motifs Revealed - Broad Institute Press Release http://www.broad.mit.edu/cgi-bin/news/display_news.cgi?id=134 Because so many genes have been conserved, or passed along from species to species throughout evolution, comparing genomes across species has emerged as a powerful tool for discovering functional elements. Comparative genomics has been extremely successful in identifying protein-coding genes and large conserved non-coding elements in human. But the majority of the non-coding elements remain largely unknown. The most elusive are small regulatory motifs, of about seven to ten base pairs, that modulate gene usage. "We set out to systematically discover an encyclopedia of regulatory motifs in the human, through the lens of evolutionary conservation," said Manolis Kellis, co-senior author of the study and associate member of the Broad Institute. "By comparing multiple species, we determined subtle conserved signals based on their repetition across the genome." These regulatory motifs define the dynamic nature of the cell, dictating which signals a gene will respond to, and which specific tissues--such as liver, heart, or muscle - a gene will be expressed in, said the researchers. "Fortunately, evolution is a good note keeper," said Kellis, who is also an assistant professor of computer science at MIT. "By referencing evolution's notes, we are now one step closer to a more thorough understanding of the human genome's controlling machinery. Once this machinery is known, we can then hopefully control the signals for medical purposes." In the March 17 issue of Nature, the Broad researchers report: * more than a hundred new regulatory motifs involved in the first stage of gene regulation, known as transcriptional initiation; and * 105 new regulatory motifs involved in post-transcriptional control, many of which are targets of microRNAs, a recently discovered mechanism of gene repression. Surprisingly, the study also led to hundreds of new microRNA genes, a number much higher than previous estimates suggested. "Nearly one-half of the motifs involved in post-transcriptional regulation are associated with microRNAs, demonstrating the extraordinary importance of this recently discovered regulatory mechanism," said Xiaohui Xie, first author of the study and a postdoctoral associate at the Broad. The researchers now estimate that at least 20 percent of genes are regulated by microRNAs, an estimate much higher than previously expected. These tiny, single-stranded pieces of RNA may be one of the principal players in regulating cellular mechanisms. MicroRNAs can inhibit transcribed messages, or interrupt a gene's ability to make protein. The study team employed an analysis method used recently by Broad scientists to study four related yeast species genomes. This work published in the May 15, 2003 issue of Nature by Kellis et. al., showed that it is possible to systematically identify both genes and regulatory elements by comparing a small number of genomes in a related species. It was unclear however whether such analyses would be possibly in the vastly more complex human genome. "Evolution is one of the most powerful tools for understanding how genes are regulated in health and disease," said Eric Lander, co-senior author of the study and the founding director of the Broad Institute. "Our ultimate goal is to use evolutionary comparison to create a comprehensive catalog of common regulatory motifs in the human genome." In addition to the authors described above, included in the study are Broad researchers Jun Lu; Edward Kulbokas; Todd Golub, who is also affiliated with Dana-Farber Cancer Institute; Vamsi Mootha, who is also affiliated with Massachusetts General Hospital and Harvard Medical School; and Kerstin Lindblad-Toh. Human gene count tumbles again http://www.broad.mit.edu/cgi-bin/news/display_news.cgi?id=4163 Estimates of the number of genes in the human genome have ranged wildly over the past two decades, from 20,000 all the way up to 150,000. By the time the working draft of the human genome was published in 2001, the best approximation stood at 35,000, yet even that number has fallen. A new analysis, one that harnesses the power of comparing genome sequences of various organisms, now reveals that the true number of human genes is about 20,500, thousands fewer than what is currently listed in human gene catalogs. The work, led by researchers at the Broad Institute of MIT and Harvard and appearing online in the November 27 issue of PNAS, has implications beyond merely settling the debate over how many genes are in the human genome. An accurate gene count can help identify the locations of genes and their functions, an important step in translating genomic information into biomedical advances. Ironically, the way genes are recognized has triggered much of the confusion over the human gene count. Scientists on the hunt for typical genes - that is, the ones that encode proteins - have traditionally set their sights on so-called open reading frames, which are long stretches of 300 or more nucleotides, or "letters" of DNA, bookended by genetic start and stop signals. This method produced the most recent gene count of roughly 25,000, but the number came under scrutiny after the 2002 publication of the mouse genome revealed that many human genes lacked mouse counterparts and vice versa. Such a discrepancy seemed suspicious in part because evolution tends to preserve gene sequences - genes, by virtue of the proteins they encode, usually serve crucial biological roles. But like it or not, the 25,000 DNA sequences were already listed in the catalogs of human protein-coding genes, and skeptics had no systematic way to remove them. "At that point, no one had gone through the gene catalogs with a fine-toothed comb to find evidence that they weren't valid," said Michele Clamp, first author of the study and senior computational biologist at the Broad Institute. Far from blatant mistakes, non-gene sequences can masquerade as true genes if they are long enough and happen by chance to fall between start and stop signals. Despite having gene-like characteristics, these open reading frames may not encode proteins. Instead, they might have other functions or possibly none at all. To distinguish such misidentified genes from true ones, the research team, led by Clamp and Broad Institute director Eric Lander, developed a method that takes advantage of another hallmark of protein-coding genes: conservation by evolution. The researchers considered genes to be valid if and only if similar sequences could be found in other mammals to nearly 22,000 genes in the Ensembl gene catalog, the analysis revealed 1,177 "orphan" DNA sequences. These orphans looked like proteins because of their open reading frames, but were not found in either the mouse or dog genomes. Although this was strong evidence that the sequences were not true protein-coding genes, it was not quite convincing enough to justify their removal from the human gene catalogs. Two other scenarios could, in fact, explain their absence from other mammalian genomes. For instance, the genes could be unique among primates, new inventions that appeared after the divergence of mouse and dog ancestors from primate ancestors. Alternatively, the genes could have been more ancient creations - present in a common mammalian ancestor - that were lost in mouse and dog lineages yet retained in humans. If either of these possibilities were true, then the orphan genes should appear in other primate genomes, in addition to our own. To explore this, the researchers compared the orphan sequences to the DNA of two primate cousins, chimpanzees and macaques. After careful genomic comparisons, the orphan genes were found to be true to their name - they were absent from both primate genomes. This evidence strengthened the case for stripping these orphans of the title, "gene." After extending the analysis to two more gene catalogs and accounting for other misclassified genes, the team's work invalidated a total of nearly 5,000 DNA sequences that had been incorrectly added to the lists of protein-coding genes, reducing the current estimate to roughly 20,500. In addition to suggesting a major revision to the human gene count, this work provides a set of rules for evaluating any future proposed additions to the human gene catalog. It also underscores the benefit of genome sequencing projects. "Without several primate genomes, we wouldn't have been able to put the final nail in the coffin of these putative genes," said Clamp. More broadly, the research reveals that little invention of genes has occurred since mammalian ancestors diverged from the non-mammalian lineage. "There's no real creativity going on in the mammalian genome," explained Clamp. That means that the number, structure, and function of protein-coding genes are not expected to differ very much from mammal to mammal, so what makes humans different from mice and dogs likely lies outside this realm of the genome. Clamp and her Broad Institute colleagues are now peering into the genomes of many other mammals, in an attempt to explain what parts of our genome truly make us human. Open|DOOR Interview (Nov 2003) http://alum.mit.edu/ne/opendoor/200311/kellis.html Manolis Kellis (Kamvysselis), SB '99, MEng '99, PhD '03, is first author on a breakthrough comparative genomics article published in "Nature". The work, completed with colleagues at the Whitehead Institute/MIT Center for Genome Research, distinguished important biological signals from surrounding nonfunctional nucleotides in yeast and has important human genome applications. What did baker's yeast, an organism that turns sugar to alcohol, teach your team about the human genome? Yeast has taught us surprisingly many lessons about the human genome. As an experimental system, it has been the model organism of choice for developing genome-wide technologies for monitoring complete cell states, simultaneously observing all genes and proteins. For our team, it became the model organism for comparative genomics. We work towards the ability to directly interpret genomic information, namely to read in a string of ACGT characters and recognize within it meaningful functional elements, such as genes and regulatory motifs that control the expression of genes. The difficulty to identify such elements comes from the fact that they are hidden amidst thousands of non-functional nucleotides. Discovering functional features is equivalent to extracting signal from noise. This is where comparative genomics comes in. Across evolutionary time, mutations in non-functional regions accumulate by genetic drift, but mutations in functional regions are selected against by natural selection. By comparing closely related species, we can thus recognize strongly conserved regions as likely to be functional, and weakly conserved regions as likely to be non-functional. Yeast proved to be a wonderful organism for comparative genomics. Its small and relatively simple genome made it feasible to completely sequence multiple relatives and align them across their complete genomes. We were for the first time able to study the conservation patterns of genes and regulatory motifs across four complete eukaryotic genomes, and develop computational methods for discovering biological signals. Finally, the extensive biological knowledge of gene function in yeast allowed us to confirm our findings and our comparative methods. We hope to apply similar methods for the understanding of the human genome, by comparing it to chimp, mouse, rat, dog, chicken, and a multitude of genomes whose sequencing is under way. This will not be an easy task due to the increased complexity of these organisms, but the lessons learned from yeast are proving to be invaluable. How are you applying your computer science expertise to comparative genomics? Computer science is playing an increasingly central role in modern biology. This has been enabled by the quantitative nature of biological data sources and efforts to represent biological knowledge in a controlled vocabulary. Additionally, technological advances have increased the ability to obtain large-scale information of cell state, leading to an explosion in both the quantity and the types of available data. Together, these transformations of modern biology have made the use of computational approaches in biology not only possible, but also imperative. The hypothesis-driven approach of specific experiments designed to answer well-posed questions is now complemented by data-driven approaches that generate hypotheses largely in silico, based on large-scale biological data. Computational tools that can discover meaningful patterns by mining through large quantities of data can be central in biological discovery. This is where a computer science background has proved to be very useful. Understanding the biology is only the first step. One has to then construct computational representations for the data at hand, develop algorithms to manipulate the resulting computational structures, define statistical tests to select meaningful results, and interpret these back into the realm of biology. To obtain sound biological results, the underlying computational methods must be sound. Each aspect of the yeast work required a strong computational component. To align the four yeast species, we developed graph-theoretic algorithms for resolving the ambiguities in the correspondence of genes and regions across the species. To identify protein-coding genes, we developed models for nucleotide change within genes and intergenic regions, and built a classifier for candidate genes according to these models. To discover regulatory motifs, we formulated statistical tests to evaluate the genome-wide conservation of sequence patterns, and built algorithms to refine these motifs into a small dictionary of regulatory elements. The marriage of computer science and biology is a necessity. My biology friends like to joke that computer science will be remembered as that little field that helped understand biology. My computer science friends instead respond: "Aah, biology! Finally a problem hard enough for computer science to solve." Good-spirited jokes aside, both parties acknowledge the birth of a field in its own right, where computation and biology combine to bring the best from both worlds. The whole makes for a rapidly moving field, where the rules are changed every few months, powerful paradigms emerge and secrets are revealed. BioIT World http://www.bio-itworld.com/archive/bases/040104.html The Search for Whole Genome Duplication Researchers at the Broad Institute have uncovered unequivocal evidence for the existence of WGD - whole genome duplication (WGD). Manolis Kellis, Bruce Biren, and Eric Lander have shown for the first time that baker's yeast (Saccharomyces cerevisiae) originated via the duplication of the entire genome of an ancestral strain; this predecessor subsequently lost some 90 percent of its genes by various means to give rise to the current S. cerevisiae genome. "This is the first time we actually see that an organism underwent complete genome duplication and went back to a single-copy state," Kellis said. WGD can occur when a cell's DNA replicates, but the cell fails to divide as normal. From an evolutionary perspective, WGD allows the newly duplicated genes to acquire new, potentially advantageous roles, or adapt to new environments. Of course, many genes are deleted or mutated, such that most traces of the widescale duplication are quickly expunged. The Broad team report in Nature that it found the missing link by sequencing the complete genome of a different yeast species, Kluyveromyces waltii, which diverged before the duplication. They showed that each region of this pre-duplication relative corresponds to exactly two regions of baker's yeast, providing definitive proof of duplication. Following deletion of 90 percent of the duplicated genes, baker's yeast returned to having one gene per function for most of the genome, ending up with only 457 additional genes, many of which are devoted to sugar metabolism. "It will be interesting to see just how far such distant echoes of genomic upheaval may be traced," the authors conclude. The study follows last year's comparative genomics tour de force by Kellis and Lander, comparing the genomes of four yeast species, also published in Nature. M. Kellis et al. " Proof and evolutionary analysis of ancient genome duplication in the yeast Saccharomyces cerevisiae. Genome News Network http://www.genomenewsnetwork.org/articles/2004/03/19/evolution.php An odd little fungus that grows in cotton seeds and a little-known species of yeast are giving scientists an idea how organisms evolve and take on diverse functions. Two new studies report that the duplication of the genome of a primitive fungus more than 100 million years ago gave rise to common baker's yeast. The studies resolve an ongoing controversy over how the common yeast Saccharomyces cerevisiae evolved. The organism is widely studied because many of the genes that control the yeast's function are also important in humans. The new findings also give scientists clues about how gene duplications can drive evolution. While a backup copy of an essential gene continues to perform needed duties, a second copy is free to mutate and take on a new role in the organism. "There have been two camps with different views of how yeast evolved," says Manolis Kellis of the Broad Institute in Cambridge, Massachusetts, who participated in one of the new studies. "Some people believe a whole genome was duplicated and others believe smaller gene clusters were duplicated. We now have the missing piece of evidence that points to whole-genome duplication." Manolis and his colleagues sequenced a species of yeast called Kluyveromyces waltii. At the same time, a team led by Peter Philippsen of the University of Basel in Switzerland, sequenced the genome of the filamentous fungus Ashbya gossypii. Both studies suggest that a genome duplication in a common descendant led to the creation of baker's yeast. Ashbya has 4,718 genes on seven chromosomes and Kluyveromyces has 5,230 genes on eight chromosomes. Duplication of the genome of their common descendant created an organism with about 10,000 genes. Over time, most of the duplicated genes were lost, but some mutated and took on new functions. In the end, the baker's yeast genome emerged with 5,714 genes on 16 chromosomes. Philippsen, who studies the fungus Ashbya, notes that baker's yeast and Ashbya have many genes in common but very different functions. Ashbya is a cotton pathogen, while baker's yeast is used to make bread. Baker's yeast grows as a single-cell organism that grows and buds off into separate cells, whereas the fungus grows as a long filament containing many nuclei. The fungus grows from one end of a filament until it runs out of nutrients, creating a branched multi-cellular organism. "These organisms have similar sets of genes, but very different lifestyles," says Philippsen. "The big challenge now is to figure out why." One clue comes from looking at some of the duplicated genes. Although most of the duplicated genes have disappeared in baker's yeast, many gene pairs remain, but have mutated to acquire different functions. For example, one gene in baker's yeast that is important for gene replication has a twin that silences, or shuts down, other genes. The researchers believe that many of these paired genes with different functions have driven evolution. "When you have duplicated genes, many of these genes will be lost over time, because you only need one to do the job," says Kellis. "But for some gene pairs, one gene has preserved the ancestral function while the other is free to evolve, sometimes taking on entirely new roles. This leads to innovation and the creation of new species." Baker's yeast provides the first clear example that whole-genome duplication plays a role in evolution. Some researchers have proposed similar events in the evolution of plants and vertebrates. "We would have to find an ancestor with a non-duplicated genome to see if similar events played a role in human evolution," says Kellis. "So far, this hasn't happened, but people are looking." Genome News Network http://www.genomenewsnetwork.org/articles/05_03/yeast.shtml Yeast Genome Revisited Ever since the yeast genome was sequenced seven years ago, researchers have debated the best way to identify the "true" genes-those DNA sequences that code for proteins. Now, researchers have sequenced three more yeast genomes and say that the current list of genes needs to be revised. By comparing the new genome sequences with the original, the researchers uncovered nearly 50 new genes and 70 stretches of DNA that regulate yeast genes. They also propose that about 500 DNA sequences previously thought to be genes should be crossed off the list. The research, published in Nature, goes far beyond bread and beer: It could serve as a model for identifying every gene in the human genome. Furthermore, many yeast genes have counterparts in humans, including some that play a role in cancer. "This study shows how valuable it is to sequence the genomes of closely related species," says Steven L. Salzberg of the Institute for Genomic Research (TIGR) in Rockville, Maryland, who wrote an accompanying News & Views article. "If we line up the genomes and see the same sequences in each species, it tells us that a gene is important." "This is just what we need to do, and in fact are doing, with the human genome," he adds. When the budding yeast, Saccharomyces cerevisiae-used to make beer and bread-was sequenced in 1996, researchers found nearly 6,000 likely genes (based on the length of the sequence and the presence of specific signals that indicate where genes begin and end). Subsequent estimates have ranged from 4,800 to 6,400 genes. According to the Nature paper, the number should be 5,538 genes. In the new study, Manolis Kellis, a graduate student in Eric S. Lander's laboratory at the Whitehead Institute in Cambridge, Massachusetts, and his colleagues analyzed the three other yeast species and compared them to S. cerevisiae. "For each possible gene sequence, we looked to see if there was evolutionary pressure to preserve that stretch of DNA," says Kellis. "We discarded about 500 sequences that were not conserved. Evolution had no reason to care about these sequences." For Kellis, the study's most exciting discovery was finding more than 70 new sequences that regulate gene activity. "We found two types of regulatory sequences," he says. "Some sequences act like little tiny traffic lights, telling the gene when to turn on and when to turn off. Others act as zip codes, or shipping addresses. They tell the cell where to send the message, once a gene is made into RNA." The researchers also found that the most variation in yeast genes occurs on the ends of chromosomes, in regions known as telomeres. Telomeres have not been completely sequenced in the human genome. "Telomeres get exchanged a lot more rapidly," says Salzberg. "My twenty-five cent bet is that the same thing is going on in humans. I would like to see telomeres sequenced in humans. This is where things are most likely to be happening, where gene rearrangements are likely to occur." He adds, "We need to finish the human sequence down to the last base." Genome Biology http://genomebiology.com/researchnews/default.asp?arx_id=gb-spotlight-20030515-01 Many yeasts win the vote Comparative genomics with two or more related species reveal limitations of single genome analysis The Human Genome Mapping Project set out to unravel the secrets of the genes by determining the primary sequence of the human genome, but it has become clear that this information is insufficient. Determination of functional and coding sequences in a primary genome sequence depends on an a priori knowledge of gene function and on statistics, and so the information obtained is incomplete and probabilistic. In the May 15 Nature, Manolis Kellis and colleagues at the Whitehead Institute/Massachusetts Institute of Technology Center for Genome Research develop and apply a general approach to determining regions of significance in primary sequence by whole genome comparison of several related species. They reasoned that evolution would conserve protein coding and regulatory elements and that comparison of more than two genomes would increase the signal:noise ratio by highlighting changes that were not due to chance (Nature 423:241-254, 2003). Kellis et al. compared the sequences of four related species of yeast, Saccharomyces cerevisiae, S. paradoxus, S. mikatae, and S. bayanus and employed a "voting system" to reach a conclusion on the validity of theoretical open reading frames (ORFs) and on the accuracy of the determination of proposed gene structures such as promoters, translation start and stop sites, and intron/exon boundaries. They propose to reduce the number of genes in the yeast gene catalogue by eliminating 503 invalid ORFs and to redefine gene structure assignments in at least 300 cases. They identified 188 genes that encode small proteins of <100 amino acids and many new genes and regulatory elements; they were also able to infer functions for more than half of their 42 newly discovered sequence motifs by categorizing the genes associated with them. In addition, they found evidence for rapid genome evolution at all of the telomeres. "The analyses will produce a substantial revision in our knowledge of the yeast genome and provide strategic directions for how we might select other sequencing targets to advance understanding of the human genome," writes Steven Salzberg of The Institute for Genomic Research in an accompanying News and Views article. "This new study of yeast genomes makes it clear that comparative genome sequencing has tremendous analytical power," he concludes. Newswire http://www.newswise.com/p/articles/view/503609/ http://www.separationsnow.com/basehtml/SepH/1,1353,6-1-1-0-0-news_detail-0-1191,00.html Yeast geneticists get a 2-for-1 deal Today's humble but extensively studied budding yeast (Saccharomyces cerevisiae) evolved when the genome of a distant ancestor became duplicated, according to a study published online by Nature this week. Manolis Kellis and colleagues studied the genetic make-up of a related yeast species, Kluyveromyces waltii, and compared it with that of S. cerevisiae. K. waltii shares a common ancestor with S. cerevisiae but diverged before the duplication event took place. The team found that key regions of the K. waltii genome are duplicated in S. cerevisiae. Originally, the entire genome was duplicated, but S. cerevisiae then evicted some 90% of its duplicated genes to make the genome fully functional. Genome duplication may boost evolutionary innovation, the authors say - duplicated genes probably supply the raw genetic material needed for new functions to emerge, and so could help organisms adapt to new environments. Whitehead Press Release http://www.wi.mit.edu/news/archives/2004/el_0308.html Study answers questions on ancestry of yeast genome CAMBRIDGE, Mass. (Mar. 8, 2004) - In work that may lead to a better understanding of genetic diseases, researchers at the Broad Institute of MIT, Harvard University and Whitehead Institute for Biomedical Research show that baker's yeast was created hundreds of millions of years ago when its ancestor temporarily became a kind of super-organism with twice the usual number of chromosomes and increased potential to evolve. The study by postdoctoral fellow and lead author Manolis Kellis of the Broad (rhymes with "code") Institute; Eric S. Lander, Broad director and Member of Whitehead Institute; and Bruce W. Birren, co-director of the Broad's sequencing and analysis program will be published online by Nature on March 7. Scientists have postulated that in a handful of instances in evolutionary history, cells may have replicated their entire genomes in events called whole genome duplication (WGD), but no definitive proof existed. The work at the Broad Institute shows conclusively for the first time that the well-studied organism baker's yeast originated through this little-understood phenomenon, resolving a long-standing controversy on the ancestry of the yeast genome. Whole genome duplication may have occurred when a cell replicated its DNA normally, as it does every time it divides, but did not split it between two resulting cells, or two cells may have fused. The result is that a yeast cell with around 5,700 genes suddenly had more than 11,000. While one copy of the gene performs its designated function, the other is free to perform a new and potentially valuable use. In addition, the organism is able to evolve more rapidly with natural selection acting on thousands of duplicated genes simultaneously, allowing for large-scale adaptation to new environments. This super-organism doesn't come without drawbacks. The excess genes cause instability in the genome and are deleted through mutation, gene loss and genomic rearrangement. As a result, millennia after the event, very few duplicated genes remain. "This is the first time we actually see that an organism underwent complete genome duplication and went back to a single-copy state," Kellis said. In the case of baker's yeast, roughly 90 percent of its duplicated genes were lost. The organism returned to having one gene per function for the vast majority of its genome, ending up with only 457 additional genes. What's the advantage to replicating the entire genome and then losing half the genes? According to one theory, by replicating the whole genome, entire systems (networks and pathways) within the organism can evolve together and take on new functions. Yeast, which metabolizes sugar and causes fermentation, apparently evolved to fill an evolutionary niche around the time that fruit-bearing plants appeared, creating an abundance of sugar in the environment. "It's the best fermenter out there," Kellis said of Saccharomyces cerevisiae, the species the group studied. Many of its surviving 457 genes are devoted to sugar metabolism. If incremental evolution over millennia is like a landscape changing through erosion, whole genome duplication is like an earthquake. "Direct study of such a cataclysmic event may provide major insights into the dynamics of genome evolution and the emergence of new functions," the authors write. Given the massive gene loss and hundreds of rearrangements, little evidence of WGD remains within the genome of baker's yeast. Tracing the development of a genome over billions of years is like printing a 5,000-page book twice without page numbers, throwing away most of the duplicate pages, shuffling both copies and binding them into a single book. Uncovering the ancestral gene order, Kellis said, would be like happening upon the original book in a hidden library. The authors found the missing link by sequencing a yeast species whose divergence precedes the duplication. They showed that each region of this pre-duplication relative corresponds to exactly two regions of baker's yeast, providing definitive proof of duplication. Researchers speculate that vertebrates, including human ancestors, may have undergone two rounds of complete duplication, but the evidence remains weak without comparison to a pre-duplication relative. Broad researchers used a new method to compare the complete genomes of each of the duplicated and pre-duplication yeast species, and they plan to apply this method to more species. Typical methods of genome comparison would "miss the genome duplication event if they focus on solely the best match for every gene and every region," Kellis said. Genomic research is leading to new understanding of the connections between different types of genetic functions and which genes were paired in our ancestors to work together. For example, uncovering the duplication event provided a new link between gene silencing and the binding of DNA-replication origins. Similarly, understanding the dynamics of genome duplication has implications in understanding disease. In certain types of cancer, for instance, cells have twice as many chromosomes as they should, and there are many other diseases linked to gene dosage and mis-regulation. "These processes are not much different from what happened in yeast," Kellis said. Whole genome duplication may have allowed other organisms besides yeast to achieve evolutionary innovations in one giant leap instead of baby steps. It may account for up to 80 percent of flowering plants species and could explain why fish are the most diverse of all vertebrates. Said the authors, "The results here suggest that it may also be fruitful to search for similar genomic signatures of WGD in other organisms. It will be interesting to see just how far such distant echoes of genomic upheaval may be traced." Kellis is also part of the MIT Computer Science and Artificial Intelligence Laboratory, and Lander is a professor of biology at MIT. The Broad Institute, known officially as the Eli and Edythe L. Broad Institute, is a research collaboration of the Massachusetts Institute of Technology, Harvard University and Whitehead Institute. The Broad's mission is to fulfill the promise of genomics for medicine. The Scientist http://www.biomedcentral.com/news/20030515/01 Yeasts get the vote Comparative genomics with two or more related species reveal limitations of single genome analysis | By Cathy Holding The Human Genome Mapping Project set out to unravel the secrets of the genes by determining the primary sequence of the human genome, but it has become clear that this information is insufficient. Determination of functional and coding sequences in a primary genome sequence depends on an a priori knowledge of gene function and on statistics, and so the information obtained is incomplete and probabilistic. In the May 15 Nature, Manolis Kellis and colleagues at the Whitehead Institute/Massachusetts Institute of Technology Center for Genome Research develop and apply a general approach to determining regions of significance in primary sequence by whole genome comparison of several related species. They reasoned that evolution would conserve protein coding and regulatory elements and that comparison of more than two genomes would increase the signal:noise ratio by highlighting changes that were not due to chance (Nature 423:241-254, 2003). Kellis et al. compared the sequences of four related species of yeast, Saccharomyces cerevisiae, S. paradoxus, S. mikatae, and S. bayanus and employed a "voting system" to reach a conclusion on the validity of theoretical open reading frames (ORFs) and on the accuracy of the determination of proposed gene structures such as promoters, translation start and stop sites, and intron/exon boundaries. They propose to reduce the number of genes in the yeast gene catalogue by eliminating 503 invalid ORFs and to redefine gene structure assignments in at least 300 cases. They identified 188 genes that encode small proteins of <100 amino acids and many new genes and regulatory elements; they were also able to infer functions for more than half of their 42 newly discovered sequence motifs by categorizing the genes associated with them. In addition, they found evidence for rapid genome evolution at all of the telomeres. "The analyses will produce a substantial revision in our knowledge of the yeast genome and provide strategic directions for how we might select other sequencing targets to advance understanding of the human genome," writes Steven Salzberg of The Institute for Genomic Research in an accompanying News and Views article. "This new study of yeast genomes makes it clear that comparative genome sequencing has tremendous analytical power," he concludes. Tufts Academic Technology First Lecture http://at.tccs.tufts.edu/events/past-events/58/ (this link contains a video of the lecture) Bioinformatics Seminar Series Computational Biology: Challenges and Opportunity To meet Tufts University's growing interest in the field of bioinformatics, AT is hosting a bioinformatics seminar series during the spring semester. The purpose of the series is to: 1) educate Tufts faculty on what is bioinformatics and how to pursue research in this area; and 2) to create a support group interested in bioinformatics and pursuing research opportunities, including collaboration and grants support. Dr. Manolis Kellis of the MIT/Broad Institute Center for Genome Research will deliver the first lecture in the series. Entitled "Computational Biology: Challenges and Opportunity," the lecture will provide an overview of the range of definitions, tools, and skills related to the term "bioinformatics." Dr. Kellis's research interest is in applying computational methods to understanding biological signals. His MIT PhD thesis focused on the computational foundation of genomics. He pioneered new methods for discovering biological signals using multiple species comparisons. Dr. Kellis currently works at the MIT/Broad Institute Center for Genome Research. A collaboration between MIT, Harvard and its affiliated hospitals, and the Whitehead Institute for Biomedical Research, the Broad Institute is a newly created biomedical research institute, aimed a realizing the human genome to revolutionize clinical medicine and to make knowledge broadly available to scientists around the world. The Center for Genome Research is an international leader in the Human Genome Project (the effort to identify all of the DNA letters that make up the instructions for a human being). The Center is the largest public sequencing center in the world, having contributed one- third of the content to the human genome sequence. Museum of Science http://www.mos.org/doc/1434 Three MIT Professors Declared "Young Innovators to Watch" at Museum of Science Next Generation Event BOSTON (November 10, 2004)-Last night, at the Museum of Science's Next Generation event, three leaders in biotechnology, computational biology and genetics announced their choices for the next generation of "young innovators to watch" in these fields. Made possible by Concord Communications, Inc. of Marlborough, MA, this year's event honored three young innovators from MIT. Chris Burge, an associate professor at MIT, was nominated by Mary Lou Pardue, Boris Magasanik Professor of Biology at MIT, developer of the fundamental geneticist's tool in-situ hybridization, and advocate for women in science. Burge physically split his laboratory to work in both the traditional "wet" and computational biology paradigms. His research includes RNA splicing and microRNAs, new developments that reveal extraordinary complexity in how genes work and suggest new disease mechanisms and cures. Chosen by Eric Lander, Founding Director of the Broad Institute of MIT and Harvard, Manolis Kellis, an assistant professor at MIT, is a principal investigator of the Computer Science and Artificial Intelligence Laboratory (CSAIL), and a member of the Broad Institute of MIT and Harvard. He introduced new computational paradigms to the complex tasks of deciphering DNA signals, understanding gene regulation, and clarifying how genomes evolve. His work may help cure disease and also contribute to our understanding of natural history and evolution. "The Museum of Science is dedicated to presenting science, technology and engineering in a variety of interesting and inspiring formats including interactive exhibits, live presentations and special events like the Next Generation," remarked President and Director, Ioannis (Yannis) Miaoulis. "We're honored to host renowned science and technology leaders and their chosen innovators-to celebrate their exciting discoveries and glimpse the future of their fields." Following a brief introduction by their mentor, each young innovator offered a 15-minute presentation on their work to date and responded to questions from the invited audience. "As a major employer in the greater Boston area, Concord Communications has benefited from the highly skilled local workforce," stated Jack Blaeser, President and CEO of Concord Communications. "As a company, we believe it is important to support the advancement of technology literacy so that Massachusetts can remain a vibrant contributor to the information economy. That is why we have chosen to become a founding sponsor of the Museum of Science's Technology Literacy Center. Our support for the Center showcases our commitment to the greater Boston community, in which we work and live." Blackwell Plant Science http://www.blackwellpublishing.com/PlantSci/latest/article.asp?id=86 Latest News - Genetic Cross-Toc: The Homologous Genes atToc33 and atToc34 Contribute to Plant Development What is the function of homologous genes in organisms? This question is often simpler to ask than to answer. In the genetically simple plant, Arabidopsis, work is underway to investigate the function of homologous genes using the wealth of data available from the genome sequence (1,2). In a recent paper in The Plant Journal, the groups of Paul Jarvis and Kenneth Keegstra published investigations into the function of a homologue of the PPI1 gene (atToc33), PPI3 (atToc34) in Arabidopsis (1).[...] Finally, it is worth noting the context of this paper. In the April 8th 2004 edition of Nature, Manolis Kellis and co-workers reported analysis of gene function in duplicated portions of the yeast genome (7). There investigations up-hold the long-standing hypothesis that when gene duplication occurs, one copy of the gene retains the original function, while the homologue diverges rapidly in sequence and function. Evidence for whole-genome duplication in Arabidopsis neatly parallels these findings and places this paper on the broader evolutionary canvass. Small fish yields big insights http://web.mit.edu/newsoffice/2004/pufferfish.html An international team of scientists, including several from the Broad Institute of MIT and Harvard, has decoded the smallest known vertebrate genome--the puffer fish or Tetraodon nigroviridis. The fish's 21 chromosomes, which together contain more than 300 million letters of DNA, tell a twisting evolutionary tale and even shed light on our own genetic makeup. Comparison with other genome sequences shows that fish proteins have diverged much faster than those in mammals, the team reports in the Oct. 21 issue of Nature. Tetraodon contains several key genes previously thought to be absent from fish. Further, comparison with the human genome suggests about 900 previously unannotated human genes. Most genes in the human DNA sequence have two counterparts in the Tetraodon genome, the researchers add, showing that the ancestors of this fish must have undergone a genome duplication at some point. Indeed, the Tetraodon sequence may even give us a window on the last common ancestor of Tetraodon and humans--a primitive bony fish that lived hundreds of millions of years ago. The Broad authors are Nicole Stange-Thomann, Evan Mauceli, Manolis Kellis, Michael Zody, Jill Mesirov, Kerstin Lindblad-Toh, Bruce Birren, Chad Nusbaum and Eric Lander. Lander is also a professor in MIT's Department of Biology. This work was supported by the Consortium National de Recherche en Genomique. The Daily Nonpareil http://www.zwire.com/site/news.cfm?newsid=11092101&BRD=2554&PAG=740&dept_id=331520&rfi=6 Yeast may rise from 11,000-gene ancestor CAMBRIDGE, Mass., Mar 08, 2004 (United Press International via COMTEX) -- Baker's yeast may have been created when its ancestor's genome was doubled, giving itself twice the capacity to evolve, U.S. researchers said Monday. MIT and Harvard researchers studied the yeast's genetic history and concluded its genome may have been created when an ancestor cell attempted to replicate its DNA during cell division. Instead of splitting the DNA between the two resulting cells, one daughter cell may have wound up with more than 11,000 genes instead of 5,700. Alhough the larger number of genes made the yeast cell more adaptable to new environments, it also created many mutations, so nearly all the original genes have been inactivated over time. "This is the first time we actually see that an organism underwent complete genome duplication and went back to single-copy state," said lead author Manolis Kellis. Most of the 457 genes left in baker's yeast are devoted to one gene function: fermentation. Other organisms, including many flowering plants, may have developed the same way, the researchers said, and the same kind of development may also explain the diverse nature of fish. Nature Highlights http://www.nature.com/nature/links/030515/030515-4.html Comparative genomics has the potential to tackle a central problem in current biological research: the identification of the functional information in the genome. The results of one of the first major contributions to comparative genomics suggest that the technique is extremely powerful and will have a major impact on genome analysis in all species including humans. Draft sequences of three yeasts separated from Saccharomyces cerevisiae by up to 20 million years of evolution were compared with the gene sequence of S. cerevisiae. The comparison yields major revisions to the gene catalogue, including elimination of 500 previously annotated genes and the discovery of 50 new ones. Nature News and Views http://www.nature.com/cgi-taf/DynaPage.taf?file=/nature/journal/v423/n6937/full/423233a_fs.html (requires subscription) Bio-IT World http://www.bio-itworld.com/archive/bases/052203.html In a tour de force of genomic technology and computational biology, researchers at the Whitehead/MIT Center for Genome Research have produced a comparative study of four strains of yeast, with dramatic implications for our understanding of gene inventory and regulatory DNA sequence motifs that direct gene expression. Manolis Kellis, Eric Lander, and colleagues sequenced the approximately 12-million basepair genomes of three yeast species n 1996. Back then, sequencing a yeast genome required hundreds of researchers and more than a year; today, a single high-throughput center blasts through the sequence in about a week. Two major results emerge from this comparative genomics study. First, Kellis and colleagues show that more than 500 putative genes predicted by algorithm approaches are spurious, dropping the total number of yeast genes below 6,000. Second, they newly identify 42 conserved regulatory sequence motifs. The lessons from this study in yeast are directly relevant to the future study and understanding of the human genome. Diario Medico http://www.diariomedico.com/edicion/noticia/0,2458,297667,00.html http://translate.google.com/translate La comparacion de genomas de levadura identifica nuevos genes La revista Nature publica hoy el analisis comparativo de la levadura Saccharomyces cerevisiae con el de otras tres especies: S. paradoxus, S. mikatae y S. bayanus. Este mitodo ha permitido identificar la funcion de elementos de la secuencia hasta ahora no identificados. Los autores consideran que el mitodo de analisis podrma aplicarse para comparar el genoma humano con el de otras especies primates. Un estudio estadounidense ha realizado un analisis comparativo del genoma de la levadura Saccharomyces cerevisiae valiindose del borrador de la secuencia genitica de otras tres especies relacionadas, Saccharomyces paradoxus, S. mikatae y S. bayanus, segzn se publica hoy en Nature. Basandose en su comparacion, el equipo de Manolis Kellis, del Centro de Investigacion Gensmica Whitehead/MIT, en Boston (Massachusetts), ha llegado a la conclusion de que 500 elementos del genoma que se pensaba que iban a ser genes, en realidad no lo son, por lo que deberman eliminarse del catalogo genitico de la Saccharomyces cerevisiae. Asimismo, deberman aqadirse 50 nuevos genes. Finalmente, la S. cerevisiae se compondrma sslo de 5.538 genes que codificarman cien aminoacidos. La identificacion de elementos funcionales codificados en un genoma es uno de los principales retos de la biologia moderna. Al contrastar estas cuatro secuencias similares, los investigadores fueron capaces de identificar secuencias del ADN de la levadura que no codificaban genes, pero que, sin embargo, podrman tener otras funciones. El equipo se ha centrado en la Saccharomyces cerevisiae por ser uno de los eucariotas mas estudiados. En una primera fase del trabajo se alinearon los genomas y se caracterizs su evolucion, definiendo las regiones y sus mecanismos de cambio. Posteriormente, se desarrollaron mitodos para la identificacion directa de genes y elementos reguladores. El analisis genitico supuso una amplia revision del catalogo genitico de la levadura, modificando aproximadamente al 15 por ciento de los genes y reduciendo el nzmero total en cerca de 500. Por su parte, gracias al analisis se identificaron de forma automatica 72 elementos del genoma, incluyendo elementos reguladores ya conocidos, asm como otros nuevos. "Se ha intentado deducir una posible funcion para cada uno de estos elementos, asm como pistas sobre las interacciones entre ellos", han especificado los investigadores de Massachusetts. Los resultados demuestran que el analisis gensmico comparativo de especies relacionadas puede identificar elementos funcionales fundamentales previamente no conocidos. Por tanto, "la secuenciacion de primates podrma proporcionar muchas pistas sobre el genoma humano", han apuntado los cientmficos. Mitodo ztil En un artmculo de opinion que acompaqa al estudio en el mismo nzmero de Nature, Steven L. Salzberg, del Instituto de Investigacion Gensmica en Rockville (Maryland), seqala que "este nuevo estudio comparativo de los genomas de la levadura aclara que la comparacion de secuencias geniticas puede tener un tremendo potencial analmtico y deductivo". Segzn el genetista, "este trabajo ofrece la posibilidad de mejorar el conocimiento de miles de genes de una sola vez, asm como obtener datos sobre la funcion de una vasta cantidad de ADN gensmico que no codifica genes. Tal y como ya se habma demostrado cuando se secuencis, la levadura nos muestra una vma hacia el mejor conocimiento de nuestra biologia". Berliner Zeitung http://www.gene.ch/genpost/2003/Jan-Jun/msg00448.html Manolis Kellis und seine Kollegen vom Center for Genome Research in Cambridge, Massachusetts wdhlten f|r ihre vergleichenden Studien die Hefearten S. paradoxus, S. bayanus und S. mikatae aus. Wie die Bierhefe besitzen sie je 16 Chromosomen. Voranalysen hatten zudem ergeben, dass die meisten der angenommenen mehr als sechstausend Erbanlagen der Bierhefe ein direktes Gegenst|ck in den drei Arten haben. F|r rund f|nfhundert dieser Gene konnte Kellis Team allerdings in keiner der untersuchten Arten eine Entsprechung finden. Die Forscher halten es daher f|r unwahrscheinlich, dass es sich bei diesen Sequenzen tatsdchlich um Gene handelt - funktionelle Bereiche also, die den Bauplan f|r Proteine beinhalten. Gleichzeitig fand das Team um Kellis durch den Sequenzvergleich allerdings 43 neue Gene, die f|r kleine Proteine mit weniger als hundert Bausteinen (Aminosduren) codieren und die bei bisherigen Analysen |bersehen worden waren. Die Forscher schdtzen die Zahl der Gene im Bierhefe-Erbgut nun auf 5 726. Whitehead Press Release http://www.broad.mit.edu/media/2003/pr_03_yeast.html Whitehead Genome Center Taps Comparative Genomics to Analyze Key Functions in Yeast CAMBRIDGE, MA, May 14, 2003 -- In another example demonstrating the power of comparative genomics, scientists at the Whitehead Institute/MIT Center for Genome Research announce that they have compared four different species of baker's yeast, the simple, age-old organism that turns sugar to alcohol, and shown that such comparisons are a powerful tool for identifying key functions in genomes. Their findings have implications for the human genome and is yet another vital step on the path to further medical and scientific discovery. The paper appears in the May 15, 2003 issue of Nature. In this project, scientists generated high-quality draft sequences of the genomes of three of the Saccharomyces yeast species, S. paradoxus, S. mikatae and S. bayanus. They lined up these genomes to that of the model organism S. cerevisiae, commonly known as baker's yeast. The resulting multiple comparisons provided a great resource for understanding the yeast genome. Highly significant is that the comparisons made it possible to more easily distinguish the "noise'' portions of the genomes--areas that appear to have little use-- from "signal," those parts that have an obvious purpose. In humans, a mere five percent of the genome is functional. "The goal is to extract important biological signals hidden in the vast noise of non-functional regions," says Manolis Kellis (Kamvysselis), a graduate student at the Whitehead/MIT Center for Genome Research and the Department of Computer Science, Massachusetts Institute of Technology, who is the first author on the paper. "These signals include genes, the building blocks of our cells, but also regulatory motifs, tiny traffic lights that turn genes on and off." Not unlike attempting separating wheat from chaff with only one's fingers, extracting signal in genomes has proved a painstaking, inaccurate process, and a frustrating one, as being able to hone in on the functions of a genome has vast implications for medical science. Yeast, a relatively simple organism, has a small, compact genome containing less noise than the human genome. It provided an excellent organism to test comparative genomic techniques. "We believe it is a good model for genome-wide comparative analysis,'' Kellis says. In principal, the approach the researchers used can be applied to any organism by choosing a set of related species to sequence and study. Overall, the scientists found their model to be a powerful tool for identifying genes and refining gene structure, rapid and slow evolutionary changes, and facets of gene regulation. "Comparative genomics is an extremely important tool. Trying to understand an ancient language like Egyptian hieroglyphs by simply looking at the words in one language may be hard. But by reading the same text in other languages like Latin or Greek and finding common structures, we can recognize words and grammar, and learn the meaning of each language. Similarly, by reading the same chapter in multiple species, we get to the basis of what is important in the book of life. Comparative genomics is the Rosetta stone of biology," says Kellis. Saccharomyces is perhaps best known as the magic that makes bread rise and fruit ferment but to scientists, it is a favorite organism of study, one that for years has helped scientists answer important questions in genetics and cell biology. Now, the availability of the three, high-quality yeast sequences means biomedical researchers can better understand these organisms, some of which mutate into invasive, deadly infections in humans. Among the findings of this analysis is that the yeast genomes hold about 5,700 genes, many fewer than the estimated 10,000 of the fruitfly and 30,000 that humans have. Because of an ancient common ancestor, humans have about 2-3,000 genes in common with the yeast, generally genes that code for basic cell machinery. Researchers were also able to identify signals controlling gene expression that typically required complex experimentation and extensive biological knowledge to find. "One of the most important results of this analysis is that regulatory motifs can be read directly from the DNA sequence," says Kellis. "When comparing multiple genomes, these signals become apparent. We now have a complete list of the most strongly conserved regulatory motifs in yeast." The four different species of yeast are as different from each other as mice are from humans, yet across the four yeast genomes, all but roughly 12 genes are held in common. In other words, a mere 12 genes or so separate one yeast species from the next. "It is striking. We saw the same thing between the human and mouse genomes. It may mean that genetic differentiation across different species is the result of very subtle events," Kellis says. Understanding what changes may turn a benign species into an invasive human pathogen will be crucial to understanding and curing disease. Studying the differences between Saccharomyces genomes offers insight as to how genes and new functions may evolve in higher organisms, including humans. "We found a small number of genes that are evolving very rapidly," says Kellis. "These are likely to be involved in speciation events." The genomes were sequenced using the Whole Genome Shotgun (WGS) approach. For each species, sequence from the entire genome was generated and reassembled by recognizing identical segments using the ARACHNE assembler, a program developed at the Whitehead Institute/MIT Genome Center. The WGS method is standard and has been successfully applied to the fruitfly and the mouse. The Saccharomyces sequences are freely available through public sequence databases and the Saccharomyces Genome Database (SGD) maintained at Stanford University. The sequence is still considered a draft because there are very small missing or ambiguous portions of the sequence. The genome of each species of Saccharomyces is about 12 million base pairs in size. The draft sequences show the order of the DNA chemical bases A, T, C, and G along the yeasts' 16 chromosomes. It includes more than 95 percent of the genomes with long, continuous stretches of overlapping DNA and represents 7-fold coverage of the genome. This means that the location of every base, or DNA letter, in the Saccharomyces genomes was determined an average of 7 times, a frequency that assures a high degree of accuracy. Today's research also represents a major step along the path of bioinformatics, a recent field of science that combines biology with computing--as not one test tube was used beyond the sequencing of the species. The project relied completely on computational analysis. "We are entering a new era where computers will provide a bigger and bigger role to the understanding of biology and genomics,'' Kellis commented. The Whitehead Institute/MIT Center for Genome Research is an international leader in the field of genomics, the study of all of the genes in an organism and how they function together in health and disease. A flagship of the Human Genome Project, the Center today houses a broad range of thriving research programs combining structural genomics, medical and population genetics and clinical medicine. The Center's annual budget is $80 million, and it employs 350 people, including scientists and medical researchers from Whitehead, MIT and Harvard. Howard Hughes Medical Institute http://www.hhmi.org/biointeractive/genomics/kamvysselis.html When Manolis Kellis was 12 years old, he and his family moved from Athens to a small town in southern France. "My dad just woke up one morning and said, 'Let's go.'" His father, who grew up in a village in Greece, wanted his children to be bilingual-a skill he regretted never having gained himself. So they hit the road. "We didn't ask questions. We just went," says Kellis, who is now a graduate student working with Eric Lander at the Whitehead Institute/MIT Center for Genome Research. "Four months later, we were living in France." The move changed Kellis's life. "In a new language and new environment I had to work really hard," he says. "And I learned that working hard is normal." It also gave him a unique and intimate appreciation of mathematics. "Learning a new language, I was able to step back and realize that words are just placeholders for meanings. They're abstractions." And the same is true in math-a discipline that is all about abstraction. "Terms like x and y have no meaning on their own," notes Kellis. "They are placeholders for other information." Four years after the move to France, Kellis was one of the top 100 students in the country. "People said, 'Oh, he's good at math because he's Greek,'" Kellis laughs. "But really it was because I had to overcome the language barrier." Although Kellis loved math, he yearned to find a practical application for his skills. So, after having learned English as a foreign language in high school, he enrolled as a computer science major at the Massachusetts Institute of Technology. There he learned to use computers to tackle a variety of problems: solving geometric surfaces in multiple dimensions, building models for human motion, and programming robots to cooperate by following simple rules, like ants in a colony. "I worked on a different problem every six months," says Kellis. Taking on diverse tasks taught him how to absorb knowledge, adapt quickly to new situations, and use his talents to do something novel. That was excellent training for the work Kellis now does with Lander, where he applies his skills as computer scientist and mathematician to learn something new about how cells work. "From an engineer's perspective, looking at life and how life works really makes sense," he says. After all, a cell is like a robot that evolution has designed and assembled. The information encoded in its DNA is like the program that runs the machine. And Kellis is working on cracking the code that cells use to live-and to evolve. Working with Lander and his colleagues at the Whitehead Institute, Kellis is comparing the genome sequences of four different species of Saccharomyces, a budding yeast that bakers and brewers have used in bread and beer for centuries. Using computer programs that he wrote himself, Kellis is searching these yeast genomes for patterns. He is specifically looking for short signals that are distributed nonrandomly throughout the DNA. Sequences that are biologically important to the survival of an organism, the researchers theorize, will be conserved by evolution. They will look the same and crop up in the same places in the genomes of different organisms-in this case, closely related species of yeast. So far the data look good. Kellis and company are finding patterns and identifying elements that appear again and again throughout the yeast genomes. Now the researchers are working on correlating these signals with their functions to understand how they regulate gene expression and enable genes to work together to build and operate a machine as dynamic as a living cell. To tease patterns from these genomes, Kellis has written thousands of lines of computer code. His days are a continuous loop of looking at data, writing programs for probing the data, and testing new ideas. "Work, observe, work, observe, work, observe," as Kellis puts it. Much of his work is actually done at home, where Kellis shuffles from bed to computer and back again-with occasional trips to the refrigerator. "The danger of working independently at home is that you'll make more trips to the fridge than you'll write lines of code," he says. Of course it also has its advantages. "You can sleep late if you worked too hard or partied too hard the night before," says Kellis. "Or you can go to the beach if it's sunny on a Thursday but rainy on Saturday." But then, it's back to work-which is fine with Kellis. "Doing a Ph.D. takes a lot of self-motivation and you have to love what you do," he explains. "But I love what I do. It's like I'm a kid still playing with toys. They're just different toys." Kellis is finishing his second year of graduate school, and if all goes well he hopes to graduate in a year or so. As for the future, Kellis is still undecided. He might want to teach, or do postdoctoral work, or maybe go out and do something totally new. Until then, he will certainly continue to work hard-and play hard, too. When he's not in the lab, Kellis enjoys getting physical: biking, in-line skating, or salsa dancing. "Being stuck in a chair all the time, my hobbies involve getting out and working my body," he says. "It's important to try to find some balance. I'm not just a brain." ============================================================ Awards: First recipient of Paris Kanellakis Fellowship http://www.eecs.mit.edu/AY98-99/announcements/9.html Manolis Kamvysselis awarded Kanellakis Fellowship at MIT May 28, 1999 The Department is pleased to announce that Mr. Manolis Kamvysselis has been chosen to receive the first Paris Kanellakis Fellowship at MIT. Mr. Kamvysselis came to MIT as a freshman in 1995, expects to complete his Master of Engineering Degree in June, 1999, and plans to continue in the Artificial Intelligence Laboratory working on his PhD. The Kanellakis Fellowship was established at MIT by General and Mrs. Eleftherios Kanellakis in memory of their son Paris who received his PhD from EECS in 1982 and died unexpectedly and tragically in 1995. The family has established two similar fellowships at Brown University where Paris was a member of the computer science faculty. Brown University has established a web page in recognition of these Fellowships: http://www.cs.brown.edu/grad/pck_fellowship.html. MIT EECS - 2003 Sprowls Doctoral Dissertation Award http://www.lcs.mit.edu/about/awards.php3 Manolis Kamvysselis and Dina Katabi receive the Sprowls Dissertation Award. The award honors the best thesis in the field of computer science, from any department at MIT. Both graduate from the department of Electrical Engineering and Computer Science. Tau Beta Pi Intercollegiate Design Competition http://www-tech.mit.edu/V117/N17/tbp.17n.html For the fourth straight year, MIT won the Tau Beta Pi National Engineering Honor Society district design competition on Saturday, beating out approximately 17 regional competitors, including Yale University, Brown University, Boston University, and Worcester Polytechnic Institute. The team of William H. Stadtlander '99, Matthew S. Duplessie '99, and Manolis E. I. Kamvysselis '99 captured the $300 first-place prize. The contest was part of the activities at the annual TBP District Convention held at the Worcester Polytechnic Institute this year. Teams designed airplane carts The team was challenged to design an airline cart that would alleviate the problem of stewards suffering back injuries while serving drinks to passengers. They were given four hours to prepare the design and a 10-minute presentation. Their idea consisted of a motorized cart that ran on a rubber mat. The mat had two grooved tracks to fit the cart's grooved wheels. The team decided to use a rubber mat so grooves would not be cut directly into the floor of the plane. The grooved track and wheels also prevented the cart from moving during turbulence. Many of the competing teams developed similar motorized-cart designs. The MIT team considered many other options, from using tubing to deliver drinks to the passengers to using a spring-based system to hang a cart from the cabin ceiling. "They really didn't give us a lot of specifications," Stadtlander said. The design problem "very open-ended" and wished the contest could have "allowed more creativity," so that the competing teams' "ideas could have been very different," Duplessie said. Brainstorming was key to success As in the local competition, the team used brainstorming and math to win. They "spent a large amount of time brainstorming to iron out the kinks in the plan," Duplessie said. They also backed up their design with dimensions and a cost analysis. "We did a significant bit of math," Duplessie said. Outfitting a 30-foot airplane with one aisle was estimated to cost about $5,000, Stadtlander said. The team also made sure to polish their presentation, since half their score was based on presentation and half was based on design. "You can come up with a great idea that works, but if you can't present it, it's worthless," Stadtlander said. The team enjoyed the competition. "I would recommend it to any freshman or sophomore next year," Duplessie said. ============================================================ Social: MIT International Celebration http://www-tech.mit.edu/V117/N22/ifair.22n.html "I-Fair has always been the most important event for ISA, just as much as it is for many international clubs on campus. It's by far the largest event, and it really fulfills the goal of ISA, which is to bring closer together the different cultures represented in MIT," said Manolis E.I. Kamvysselis '99, president of ISA. "I-Fair was a huge success this year. It's been the biggest I-Fair we've had so far," Kamvysselis said. About 2,000 people dropped by the event, and over 118 countries were represented. A record number of 43 clubs hosted booths and 27 groups put on performances, he said. "I think it was flawless this year; the spirit was there. The people love it because they see many clubs; clubs love it because they get a chance to perform," Kamvysselis said. "The I-Fair is a great opportunity for the clubs at MIT to display their traditional cultures through dance, food, and music. This year's show went really well, we had a lot of people who came who aren't from MIT, such as Harvard [University] and Boston College," said Manas D. Ratha '99, treasurer of the ISA. While some expressed concern about the chilly weather, it was not enough to the keep people from turning out for the fair. "The weather could have been a bit warmer and sunnier, but it cooperated quite well, considering" that weather services had forecasted rain that day,Kamvysselis said. Besides the spring I-Fair, ISAalso hosts a "mini I-Fair" in the fall, but "we don't want to make it as big as I-Fair. I-Fair originated in the spring, when the good weather begins; it's sunny, and people have more time to perform," Kamvysselis said. "I-Fair is an event unique in the life of MITstudents. It's the one and only event that brings together so many students from so many different backgrounds and interests and lets them participate actively in the event, [giving] them a chance to show a bit of themselves to such a wide and diverse public," Kamvysselis said. ============================================================ Implementation of exon/intron evolution: http://pages.cpsc.ucalgary.ca/~gaoj/research/courses/biocomp/ In computer science, evolutionary computation is an important computational paradigm that comes from the nature. It tries to use evolution mechanisms existing in the nature to solve computer problems. Therefore new discoveries and hypothesis on the natural evolution could give new ideas to evolutionary computation. According to the known facts, in creatures' DNAs there are large quantities of genes that are not ``translated'' by the creatures into the phenotypes, and they are sometimes called ``garbage genes''. But most people, including me, think they are not garbages at all. Their functions are simply unknown currently. So many people use the term introns to refer to such function-unknown genes instead of ``garbages''. Correspondingly, the known genes are called exons. Many hypothesis are made on these introns. People think they could be meta data of the exons, or the records of the whole evolution histroy[Kam]. In January, a paper in Nature with the title Loss and Recovery of Wings in Stick Insects[WBM03] reported a new discovery in evolution -- some species can lose a complex organ and get it back some time later. This phenomenon may happen for several times during the evolution. It amazes scientists a lot because it reveals a fact that some complex functions of a species can be temporarily disabled and latent in the genes and be triggered by the environment again some generations later to come back to the species. This discovery interests me greatly. Associating the introns facts and the phenomenon revealed by the paper, I come to the hypothesis that these genes are turned from exons into introns -- thus disabled temporarily and still in the genes, and later turned from introns back to exons -- enabled again. It seems that such a mechanism can probably increase the adaptability of creatures, especially in an ever-changing environment. A natural thought is to test whether such a mechanism can result in increase of adaptability in evolutionary computation. Therefore my idea for this project comes into being -- a Genetic Programming with introns and exons. In this report I explain the experiments on the Genetic Programming with introns and exons, and show the results I got: Section 2 presents the problem selected for the Genetic Programming to solve; the implementation is discussed in Section 3; Section 4 explains the experiments I made, as well as the results I got from the experiments; finally in Section 5 and 6, the report is concluded and future work is indicated.