Research Plans
Robust, interpretable, and scalable methods for single-cell comparative multi-omics analysis
Background: Rapid developments in single-cell genomic technologies have revolutionized the resolution, depth, and complexity at which biological systems are being investigated, marking a new era in biology at scale. These technologies allow us to capture the genome, transcriptome, epigenome, proteome, immune repertoire, and spatial organization of complex tissues, organs, and whole organisms, one cell at a time, generating comprehensive atlases across multiple organisms. The broad impact and wide-spread adoption of single-cell technologies have reshaped life sciences, an achievement that was recognized by Science magazine as the breakthrough of the year in 2018.
These technological advances have been complemented by methodological developments to address significant computational challenges associated with single-cell analysis, including the scale, sparsity, and high level of noise inherent to these datasets. However, the majority of progress in the field has been focused on single-cell transcriptomic profiles, while alternative data modalities are relatively under-explored. Furthermore, tools for comparative analysis of cell states across different conditions while accounting for the underlying topology of cell state landscapes remain largely unexplored. Grounded in and driven by my prior work on multi-resolution single-cell state characterization (ACTION / ACTIONet frameworks), I aim to develop a series of novel methods and models to characterize, prune, and align condition-specific cell states using multiple, complementary -omic profiles. Specifically, I propose the following aims (Figure 1).
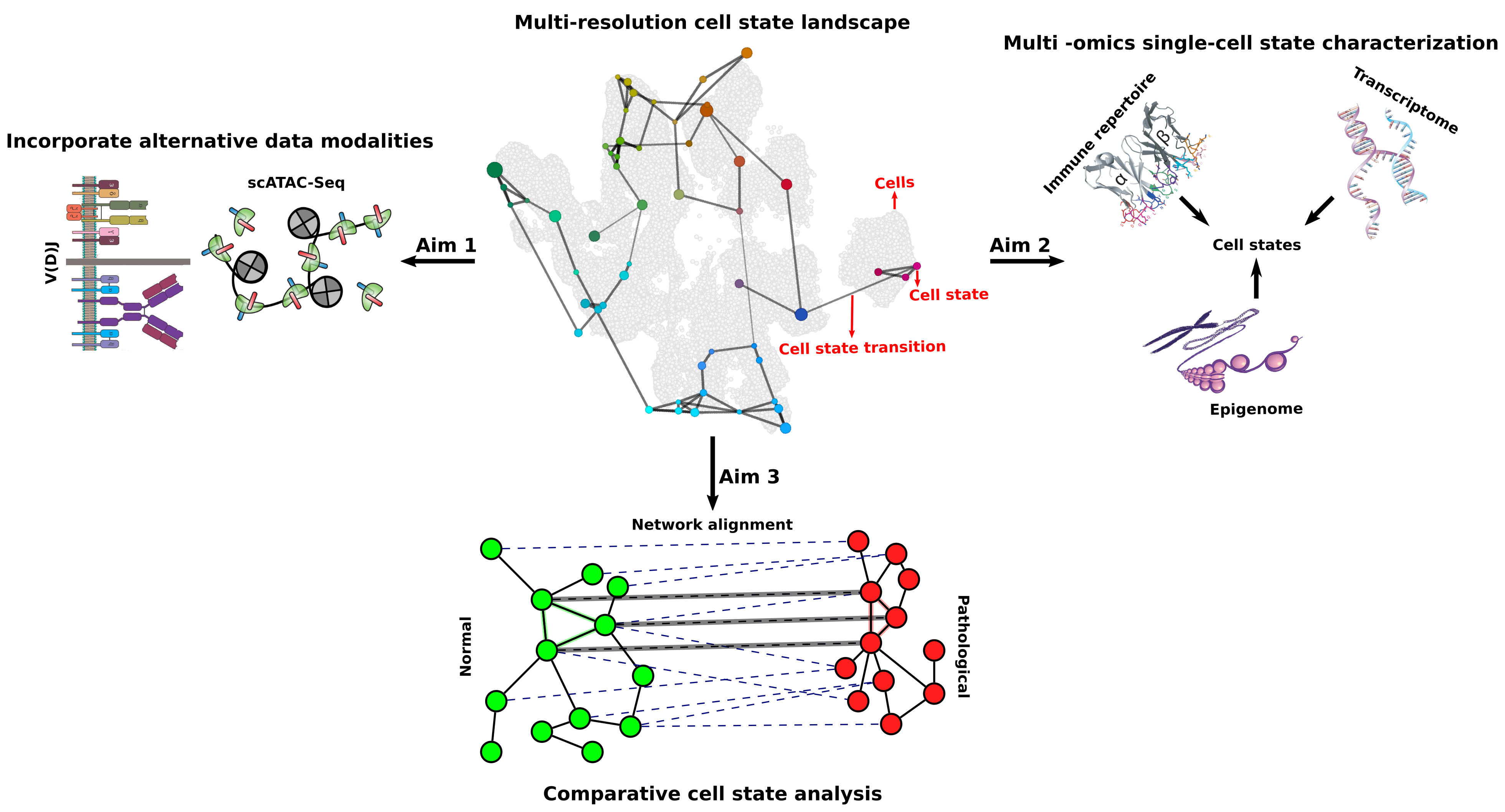
Aim 1. Incorporate alternative data modalities to characterize single-cell states
Aim 1.1 Construct a suitable metric space for analysis of single-cell ATAC-seq profiles. Overview: Single-cell ATAC-Seq (scATAC-Seq) is one of the most widely used epigenomic assays that is designed to profile chromatin accessibility. This is of significant interest as the accessibility of chromatin plays a crucial role in controlling genomic regulatory elements, thus shaping the dynamics of the regulatory landscape in health and disease. Recent developments have allowed high-throughput measurement of chromatin accessibility in hundreds of thousands of cells. Analyzing single-cell epigenomic datasets, however, is particularly challenging due to the excessive sparsity of input data (due to dropouts, as well as the limited copies of DNA molecules per cell), lack of a reference features set, and significant differences in the sparsity of different genomic regions and cells.
Approach: I will develop a comprehensive framework (atACTION) to construct a robust and reproducible representation of the single-cell epigenomic landscape. The core of atACTION is a novel approach that adjusts for significant differences in the sparsity pattern of different cells and corrects for the informativeness of ubiquitously accessible versus dynamically regulated chromatin regions. To this end, atACTION uses a series of rank-1 updates of the accessibility matrix to adjust for the sparsity of peaks, cells, and informativeness of genomic locations, resulting in a rank-k perturbation of the original matrix. Using a fast randomized algorithm for computing the SVD of sparse matrices, coupled with existing theoretical foundation for updating SVD of rank-k perturbed matrices, I will develop efficient techniques to implicitly compute and reduce the adjusted accessibility matrix, which is then used as input to the ACTIONet framework for multi-resolution decomposition of the epigenomic profiles.
Aim 1.2 Construct a continuous landscape to capture biophysical relationships between immune cell receptors of single-cells
Overview: The diversity and specificity of immune cell receptors play a fundamental role in regulating their adaptive response to intrinsic and extrinsic threats, including viral infections and cancers, and its dysregulation plays a significant role in the development of autoimmune disorders. Traditional methods for analyzing immune repertoire are based on classifying immune cell receptors into disjoint groups to identify “clonotypes” that could then be traced back to a potential history of clonal expansions. However, this approach does not consider the biophysical properties of receptors, which directly contribute to their binding affinity, nor does it consider the relationship between similar, but not identical immune receptors.
Approach: I will use multiple sequence alignment to match receptors from different immune cells. To define descriptors that best represent the binding affinity, I will focus on the complementarity determining regions (CDRs), which are hypervariable regions of the receptors and play a key role in determining its binding specificities. I will then encode aligned sequences using E-descriptors, which are defined based on 237 amino-acid physical-chemical properties. This will effectively map immune cell receptors into a quantitative, suitably normalized space, in which proximity of receptors reflects their biophysical similarity, which is hypothesized to be directly related to their binding affinity. I will then use these descriptors as input to the ACTIONet framework.
Aim 2. Leverage emerging single-cell multi-omics technologies to decouple shared and omic-specific aspects of the cell state landscape.
Overview: The systems-level behavior of a cell is driven by a complex network of interactions between different biomolecules, including DNA, RNA, and proteins. This intricate machinery is regulated at various levels of control, including the expression and availability of receptor proteins, intermediate signaling pathways, and downstream transcription factors, as well as epigenomic control and accessibility of regulatory elements. To fully understand and untangle the causal factors and processes that drive decisions in development, health, and disease, it is of paramount importance to simultaneously integrate information at the different -omic layers. Using the latest developments in single-cell profiling technologies, it is now becoming possible to simultaneously measure multiple -omic layers, including proteins, transcriptomes, immune repertoire, and CRISPR perturbations, as well as joint profiling of epigenomic and transcriptomic profiles, in the same single cell. However, scalable methods to simultaneously incorporate multiple -omic layers while accounting for unique sources of noise inherent to different single-cell profiling technologies are still lacking.
Approach: I will generalize the ACTION decomposition technique to multi-omics datasets, providing the mathematical foundation for multi-view single-cell state discovery. ACTION combines Archetypal Analysis (AA), a highly interpretable decomposition technique, with separable Nonnegative Matrix Factorization (sepNMF), a restricted subclass of NMF with a global convergence guarantee, to develop a novel decomposition technique that inherits the reproducibility and better convergence properties of sepNMF, while still benefiting from the high interpretability of AA. To extend this end, each single-cell -omic profile is first independently factorized. Integration between different -omics layers is accomplished by adding a constraint that enforces latent representations to be close to a common consensus subspace that captures the shared underlying manifold.
Aim 3. Develop a comparative analysis framework for cell state mapping across species, phenotypes, and individuals.
Overview: The first generation of single-cell atlases provides a blueprint of the identify, dynamic, and organization of single-cell states within healthy tissues. To better understand how deviations from these normal states can contribute to the onset and progression of different disorders, a second generation of studies aim to measure single-cell profiles from coupled healthy/disease conditions, including neurodegenerative, neurodevelopmental, and neuroinflammatory disorders, as well as different cancers and autoimmune disorders. The traditional approach to analyzing these datasets is to usebatch-correction prior to integration. However, batch-correction is primarily driven by the dominant signal coming from the cell-type/state difference, thus it is susceptible to removing subtle cell state perturbations that are associated with the onset and development of pathology.
Approach: Given a pair of independently analyzed single-cell profiles, I will develop a comparative analysis framework to align their corresponding cell states while preserving the underlying topology of each cell state. Grounded in my previous work in extending the state-of-the-art of global network alignment algorithms, I seek to develop a sparse network aligner to identify conserved regions between the pair of cell state graphs. Aligned scores are then used to build a statistical framework to assess the significance of alignment and distinguish matched/unperturbed, matched/perturbed, and mismatched (depleted/emerged) cell states.
