EpiMap Repository
Overview
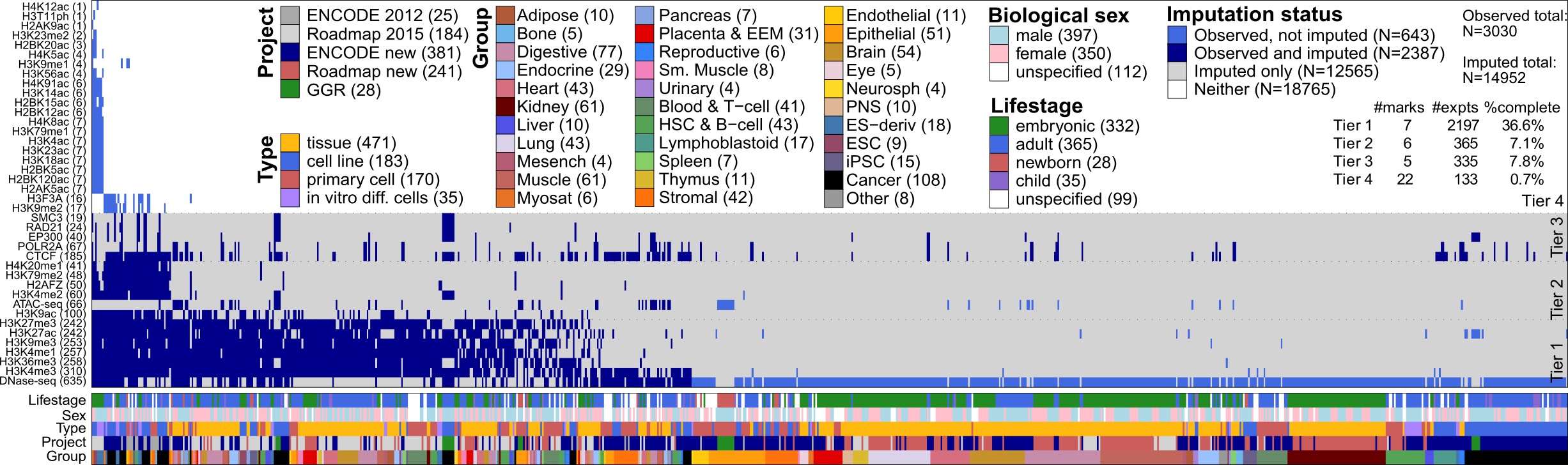
We aggregated and uniformly re-processed 3,030 references from ENCODE, Roadmap, and GGR, and imputed 14,952 datasets across 859 biosamples and 18 marks/assays, which we report here as EpiMap (for Epigenome Integration across Multiple Annotation Projects):
In addition to tracks, visualization, and metadata, we make results from gene regulation and disease dissection analyses available interactively at http://compbio2.mit.edu/epimap_vis/.
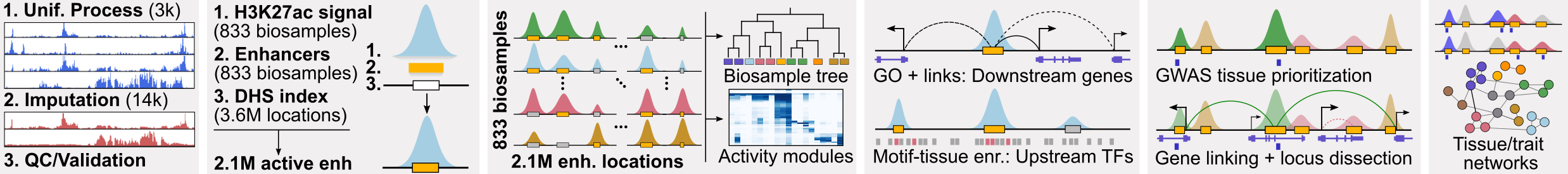
Paper: Boix et. al (2021) - Regulatory genomic circuitry of human disease loci by integrative epigenomics
Contact Carles Boix - cboix at mit dot edu
Tracks Download and Visualization
Tracks and Metadata
- Biosample metadata table (xlsx, tsv, html summary)
- Track download (WUSTL Browser)
- Average per-group and per-mark tracks (bigWig files)
- Track visualization on the WUSTL Browser
Track Hubs
We provide track hubs for visualization of all bigWig datasets, both on the UCSC browser and the WUSTL browser. Due to the size of the dataset, we have created track hubs of different sizes and at various levels of resolution.
- One huge hub for the UCSC Browser and the WUSTL Browser. (These full hubs are very large (17k+ tracks), only use them if you want to load tracks from the full dataset simultaneously.)
- Average per-group and per-mark tracks hub for the UCSC Browser and the WUSTL Browser
- Per-sample group trackhubs: directory (e.g. if you want to see all Endothelial samples)
- Custom track hubs through an interactive website where you can select specific biosamples and assays to export to WUSTL/UCSC.
Chromatin States
We provide chromatin states (ChromHMM) for each of the 833 biosamples. These are calculated using the 18-state Roadmap model from observed and imputed tracks of six histone marks (H3K27ac, H3K4me1, H3K4me3, H3K36me3, H3K9me3, H3K27me3).
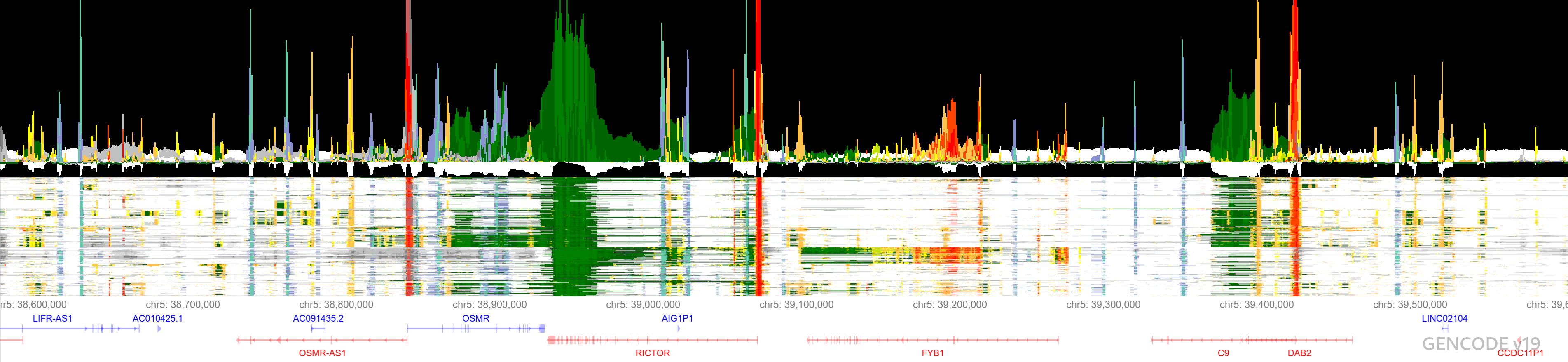
Interactive Browser
In addition, we have created an interactive browser to enable exploring the following data and analyses interactively:
- Tracks and metadata (allows export + custom trackhubs)
- Enhancer modules + gene sets + transcription factor enrichments and networks
- GWAS enrichments and tissue prioritzation, gene-enhancer links, and GWAS locus visualizations
Code
Code for the interactive browser is available on github at https://github.com/cboix/epimap_vis
We host this app online, but you can also run the interactive browser on your computer using the shiny library in R:
# Install shiny and other required packages:
pkgs = c('cba', 'DT', 'heatmaply', 'igraph', 'markdown', 'RColorBrewer',
'shiny', 'shinycssloaders', 'shinydashboard', 'shinyjs', 'shinythemes', 'shinyTree', 'shinyWidgets',
'stringr', 'tidyr', 'visNetwork')
# Check for missing packages:
new.pkgs <- pkgs[!(pkgs %in% installed.packages()[,"Package"])]
# Install the missing packages:
if (length(new.pkgs)) install.packages(new.pkgs, dependencies=TRUE)
# Download and run the app from github:
library(shiny)
runGitHub("epimap_vis", "cboix")
In addition, we have manuscript analysis code on github at https://github.com/cboix/epimap_analysis
Analysis Datasets
Datasets corresponding to manuscript analyses can be found here and are summarized in the following table:
| Dataset | Files/Locations | Description |
|---|---|---|
| Imputation metadata | Tables in xlsx, tsv, summarized in html, and directory | Extended tables matching manuscript analyses. For most use cases, the main metadata spreadsheet describes biosamples, attribution to ENCODE/Roadmap, and flagged samples. |
| DHS list | website | 3.5M+ DNaseI hypersensitive sites from Meuleman et al., (2020), which we overlay with H3K27ac activity and ChromHMM enhancer and promoter states to define active enhancers and promoters |
| Enh./Prom. locations | directory, enhancers (0-ind), promoters (0-ind); enh. matrix hdf5, mtx; prom. matrix hdf5, mtx | Enhancer, promoter, and dyadic element locations, enhancer/promoter + H3K27ac intersections as sparse matrices, and mappings from element lists to DHSs |
| Mark matrices | H3K27ac, H3K4me1, H3K4me2, H3K4me3, H3K9ac, DNase-seq, column names, directory | Dense mark activity matrices (3.5M+ DHSs x 833 biosamples) in hdf5 format. Loadable with h5py in Python or rhdf5 in R. |
| Enhancer modules | assignments, centers, directory | 300 modules for active enhancers defined by k-centroids clustering. We provide cluster definitions (centers) and enhancer assignments to the clusters. |
| Module enrichments | archetype motifs, full motifs, GO terms | Enrichments on 300 modules for GO terms (GREAT) and motifs (JASPAR 2018, HOCOMOCOv11, Jolma 2013 HT-SELEX). Motifs are split into archetypes according to Vierstra et al. (2020) |
| RNA-seq datasets | Matrices (matrix-market format): all, full, pc, full + normalized, pc + normalized, id mapping | 341 RNA-seq datasets from ENCODE matched to the unique epigenome identifiers. We provide all genes and only protein coding genes, as well as raw and quantile-normalized matrices, and the mapping between epigenome IDs and ENCODE accessions. |
| Gene-Enh. Links | per-sample directory or per-group directory | Per-sample and per-group gene-enhancer links by mark activity-by-gene expression correlation. Per-group links represent averages of all samples from a specific sample group. |
| GWAS enrichments (original) | See note below: Original enr. (tsv (1%), tsv (0.1%), Rda); summary figs. and GWAS loci figs.; enh. (tsv, Rda) and links (tsv, Rda) | GWAS enrichments resources, including enrichments, summary figures, GWAS loci figures (all plotted links in GWAS loci are given per-folder as either tsv.gz or Rda files), and lead SNP-overlapping enhancers and links. |
| GWAS enrichments (revised) | Revised enrichments for epigenomes (tsv, Rds); modules (tsv, Rds); and tree nodes (tsv); summary figs. and GWAS loci figs.; enh. (tsv, Rda) and links (tsv, Rda) | GWAS enrichments resources, including enrichments, summary figures, GWAS loci figures (all plotted links in GWAS loci are given per-folder as either tsv.gz or Rda files), and lead SNP-overlapping enhancers and links. |
Note: We have updated the site to correct for two mistakes in the GWAS enrichment analysis:
- The first mistake was that we calculated the enrichment in the wrong direction: instead of calculating the enrichment of SNPs in biosample enhancers as was previously done in Roadmap, we mistakenly calculated the enrichment of enhancers in GWAS lead SNPs. This led to double-counting SNPs and inflated enrichment statistics in the case of multiple nearby enhancers within 2.5kb of the same lead SNP, which were all counted as independently overlapping the same SNP, when in fact the multiple overlaps came from their genomic clustering in the same locus.
- The second mistake was that our FDR procedure only controlled for inflation due to multiple enhancers proximal to the same SNP, but not for the multiple hypothesis testing across many tissue-trait combinations, and thus our original reported statistics correspond to nominal P-values rather than FDR-corrected P-values. Empirical FDR estimates for all of our original enrichment analyses are: 38% for epigenomes, 80% for modules, 55% for tree nodes.
We have re-calculated our enrichments (tsv, Rds) with a snp-centric hypergeometric test as in Roadmap Epigenomics (2015) to better control FDR. For epigenomes, these are enriched for 226 GWAS at FDR of 1% (256 at 2%, 325 at 5%). Please contact cboix at mit dot edu with any questions.
We also provide updated results for the modules (tsv, Rds) and tree nodes (tsv) analyses and have fully updated the interactive browser to add the revised results for all visualizations. Finally, we re-created the majority of our paper’s GWAS enrichment figures using the revised analysis, which can be downloaded here (pdf) and includes panels on empirical FDR.